
The company noted the phase 3 study looked at MicroLine as a potential on-demand treatment for presbyopia.

The company noted the phase 3 study looked at MicroLine as a potential on-demand treatment for presbyopia.

According to Penny Asbell, MD, FACS, MBA, the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) surveillance study has identified high levels of in vitro antibiotic resistance among ocular staphylococcal and pneumococcal pathogens, resistance that may affect treatment success. She discussed the program at the American Academy of Ophthalmology’s 2022 annual meeting in Chicago.

At AAO, Kamran Riaz, MD, discussed the new textbook, "Optics for the New Millennium," which is intended to be a compendium resource for ophthalmologists.

Harvey Uy, MD, has been using the Ally adaptive cataract treatment system at his clinic in the Philippines, and it is allowing him to be more efficient.

Jeff Nau, PhD, MMS, President and CEO of Oyster Point Pharmaceuticals, provides an appraisal of the company's pipeline as well as updates on recent innovations to hit the market.

According to a BBC News report, the woman showered while wearing her lenses and she contracted a rare parasite infection called Acanthamoeba keratitis.

According to a news release, Oculis officials hope to forward product candidates to address areas of significant medical needs, including diabetic macular edema, dry eye disease, and neuro-retina indications such as glaucoma, affecting growing patient populations.

The company noted that testing of a commercial supply of the implants, which included exposing them to multiple punctures with a needle, found they did not meet their standards. The company has notified the FDA, and is working with the agency on the recall process.

According to a news release, Eyenuk will use the capital to expand its AI product platform with additional disease indications and advanced care coordination, and to accelerate the platform’s global commercialization and adoption.

According to the company, latanoprost ophthalmic solution 0.005% successfully met the primary and all secondary endpoints, and it plans to file an NDA with the FDA in Q1 2023.

Meghan Berkenstock, MD, presented a paper at AAO titled “Effectiveness of Durezol as Additional Agent to Systemic Medications for Treatment of Scleritis,” and shared the highlights in a conversation with Ophthalmology Times.®

A California ophthalmologist posted a video of a procedure she performed to remove 23 contact lenses from a woman’s eye, and the video has gone viral, with more than 1 million views.
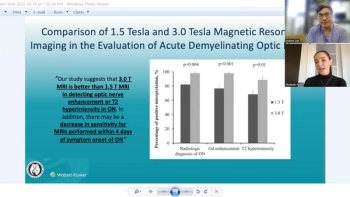
On this week's episode of the NeuroOp Guru, Andy Lee, MD, and Elizabeth Fortin, MD, compare the 1.5 Tesla magnet and the 3.0 Tesla magnet for MRI.

Kathryn A. Colby, MD, PhD, discusses highlights of the World Cornea Congress, which was held recently in Chicago.

NovaSight’s CureSight is a digital device that proved in a pivotal study to be non-inferior to patching, according to the company.

The training will overlap with World Sight Day, which spotlights the importance of eye care. Orbis's clinical staff and medical experts along with UC Davis Medical Health physicians and staff will share their knowledge with nearly 50 ophthalmologists, ophthalmology residents, nurses, and biomedical engineers from Bolivia, Chile, and Peru.
Nanoscope Therapeutics Inc. announced the FDA has granted Fast Track Designation to MCO-010, an ambient-light activatable multi-characteristic opsin optogenetic monotherapy to restore vision in blind patients, for the treatment of retinitis pigmentosa via intravitreal injection.

Shawn O'Neil of ViaLase shares updates on the company's femtosecond laser, image-guided, high-precision trabeculotomy (FLigHT) treatment.

At AAO 2022, Mitch Shultz, MD, provides discourse on iStent inject vs Hydrus contralateral eye evaluation data.

Adam Szaronos, President and CEO of Trukera Medical, discusses the recent rebranding of TearLab as well as the launch of the ScoutPro Osmolarity System.

According to a study by researchers at the Scheie Eye Institute in the Perelman School of Medicine at the University of Pennsylvania, patients’ low-light sensitivity improved by factors of thousands in a clinical trial.

A team of National Institutes of Health scientists have shed light on how genetic architecture determines gene expression, tissue-specific function, and disease phenotype in blinding diseases.
According to data released at the American Academy of Ophthalmology's 2022 annual meeting, a large number of wet AMD patients do not return for treatment.

Nanoscope Technologies LLC announced this week it has received Direct Phase II SBIR grant from National Institutes of Health for developing an innovative approach to autonomously regulate pressure for treatment of Glaucoma in a gene-agnostic manner.

UCI team demonstrates the adult brain has the potential to partially recover from inherited blindness.

One of the country’s leading ophthalmologists, Steven Schallhorn, MD, says his life and career have been shaped by his time as a Navy pilot in the elite Top Gun program.
Surrozen Inc. announced this week that it has entered into a collaboration and license agreement with Boehringer Ingelheim to research and develop SZN-413 for the treatment of retinal diseases.
Data from the Phase 4 observational real-world safety study of the intravitreal implant were presented at the annual American Academy of Ophthalmology’s 2022 annual conference in Chicago.
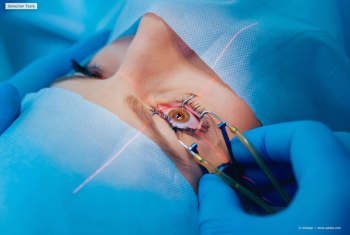
According to investigators, ophthalmologists may be able to safely cut back on having anesthesiologists or nurse anesthetists routinely at bedside during cataract surgery.

Visiox Pharma has announced the submission of a New Drug Application to the FDA for lead product candidate, PDP-716.