
Provectus will have an exclusive worldwide license of intellectual property developed by the Ophthalmic Biophysics Center of the Bascom Palmer Eye Institute.

Provectus will have an exclusive worldwide license of intellectual property developed by the Ophthalmic Biophysics Center of the Bascom Palmer Eye Institute.

At the recent Women in Ophthalmology Summer Symposium, held in Monterey, California, Cathleen M. McCabe, MD, outlined how pilocarpine HCl Ophthalmic Solution 1.25%, the first and only FDA-approved eye drop to treat presbyopia could offer an opportunity for patients looking for spectacle independence.

The clinical trial is examining the efficacy of two doses of UBX1325 compared to every other month treatment with aflibercept through 24 weeks.

According to Nicox SA, the patients made their final 3-month visit last week. Topline results of the trial are expected in November.
Faricimab simultaneously targets and inhibits two disease pathways involving Ang-2 and VEGF-A linked to a number of vision-threatening retinal conditions.

The Eye Center at UC Davis Health has two new endowed chairs focused on glaucoma, thanks to a $4 million donation from business entrepreneur Daryl Geweke.

In AMD research, the experimental animals used are often young male mice. This is not optimal for the development of new treatments, as the disease most often affects the elderly – and women.

Claes H. Dohlman, MD, PhD, widely considered the father of modern cornea science, is a recipient of the 2022 António Champalimaud Vision Award. The award, considered the “Nobel Prize of Vision," comes with a $1 million prize .

LUNA trial will evaluate the 2x10^11 vg/eye (2E11) and a new lower 6x10^10 vg/eye (6E10) dose of Ixo-vec, with enhanced prophylactic steroid regimens in patients requiring frequent anti-VEGF injections. Interim data anticipated throughout 2023.

Heru debuts dark adaptation, the newest modality added to its wearable health and wellness platform’s vision screening line-up.

Medication is administered after LASIK and injectable prophylaxis during cataract surgery.


Sigma extends access to powerful no-code cloudscale data analytics for industry-compliant data cloud so healthcare professionals and researchers can find faster answers to critical questions.

Nanoscope Therapeutics’ Phase 2 STARLIGHT open-label trial enrolled 6 subjects with advanced vision loss due to a clinical or genetic diagnosis of Stargardt disease.
The inflationary economy and lasting effects of COVID-19 have caused staff and product shortages, a rise in labor costs, and increased patient sensitivity to medication costs.

The ScoutPro system allows eye care practices to minimize tech time and seamlessly integrate osmolarity testing into any practice configuration or patient protocol.

According to the company, the NDA submission for Nyxol eye drops in first indication is on track for late 2022.

Haiyan Gong, MD, PhD, professor of ophthalmology and anatomy and neurobiology at Boston University School of Medicine, has received $200,000 through a Standard Award in National Glaucoma Research from the BrightFocus Foundation.
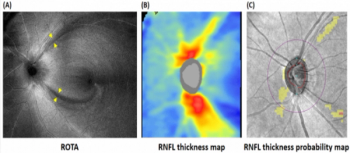
A research team led by the Department of Ophthalmology, School of Clinical Medicine, LKS Faculty of Medicine of The University of Hong Kong, with collaborators from the Faculty of Medicine of The Chinese University of Hong Kong and local and international partners, have developed a new technology ROTA to unveil the optical texture and trajectories of the axonal fiber bundles on the retina.

Zeiss parlays technology into standardization for ophthalmologists and their patients
Scientist have proposed several methods for converting stem cells into RPE, but there is still a gap in our knowledge of how cells respond to these stimuli over time.

Oral ZPX3330 found to demonstrate favorable safety and tolerability profile in interim masked safety results, consistent with 11 Prior Trials of APX3330.

According to the company, the Phase 3 pivotal trials met pre-specified primary efficacy endpoints for both doses of iDose TR., supported an anticipated upcoming NDA submission.

Regeneron announced that the primary endpoints were met in two pivotal trials investigating novel aflibercept 8 mg with 12- and 16-week dosing regimens in patients with diabetic macular edema and wet age-related macular degeneration.

Timing of type 2 diabetes or hypertension diagnosis may impact the risk of primary open-angle glaucoma.

Craig Morgan, MD, and Eye Consultants of Huntington, West Virginia, paid $907,074.64 to resolve allegations that they submitted false claims to Medicare and Medicaid, the U.S. Attorney’s Office for the Southern District of West Virginia reported.

The first stage of the study will evaluate the safety and relative pharmacodynamic effect of different doses of subcutaneously administered D-4517.2 compared to intravitreal injection of aflibercept, an approved therapy, in both wet AMD and DME patients up to 12 weeks.

If approved, TP-03 may offer treatment for millions of patients with Demodex blepharitis. TP-03 is now also being studied for the treatment of Meibomian Gland Disease in patients with Demodex mites.
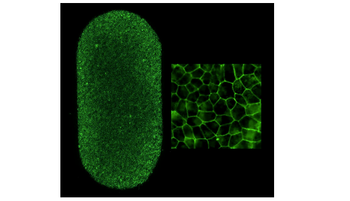
According to the National Institutes of Health, the therapy was derived from the patient’s blood by converting blood cells to iPS cells which were then programmed to become retinal pigment epithelial cells, which were surgically implanted as a patch of tissue.
GATHER2 met its prespecified primary endpoint of mean rate of growth (slope) in GA area at 12 months with statistical significance and a favorable safety profile. With this data in hand, Iveric Bio is working to submit a New Drug Application for Zimura to the FDA by the end of the first quarter of next year.