Biosimilars
Latest News
Video Series
Latest Videos
CME Content
More News


Included in the agreement are ranibizumab-nuna 0.05 mL injection (BYOOVIZ), referencing LUCENTIS (ranibizumab), and aflibercept-yszy 0.05 mL injection (OPUVIZ), referencing EYLEA (aflibercept).
Yesafili is the first biosimilar to Eylea to be approved by Health Canada.

FYB203 has been approved by the US FDA and UK Medicines and Healthcare products Regulatory Agency for the treatment of neovascular age-related macular degeneration (nAMD), diabetic macular edema, macular edema following retinal vein occlusion, and visual impairment due to myopic choroidal neovascularization.

This agreement allows a launch in the US in the second half of 2026 or earlier in certain circumstances.
The biosimilar to aflibercept (Eylea) had already received approval in the EU and US.
AVT06 is Alvotech’s proposed biosimilar to Eylea (aflibercept) 2mg.

The two products, both manufactured by Amgen, are aflibercept biosimilars Pavblu and Skojoy. Both biosimilars are indicated for treatment of AMD.

Previously, Teva was established as the European commercialisation partner of FYB201, Formycon's biosimilar to ranibizumab (Lucentis).

The EMA issued a positive opinion and recommendation for marketing authorization for Eydenzelt (biosimilar aflibercept).
The biosimilar now known by the brand name OPUVIZ was previously known as the biosimilar candidate SB15.

Afqlir (aflibercept) is a 2 mg vial kit and pre-filled syringe for intravitreal injection.
Visual acuity and macular anatomy were maintained, with some changes in fluid management.

Aflibercept biosimilars have cleared several hurdles on their way to marketplace

LUBT010 is a ranibizumab biosimilar to Lucentis and the study achieved its primary endpoint

The biosimilar was approved for treating patients with age-related neovascular (wet) macular degeneration (nAMD) and other serious retinal diseases.

The FDA recently approved Biologics' Yesafili as well as Samsung Bioepis and Biogen's Opuviz, both close copies of Regeneron Pharmaceutical’s Eylea. The approval offers ophthalmologists and patients additional therapeutic options.
The agency approved Yesafili (Biocon Biologics) and Opuviz (Samsung Bioepis, Biogen) as biosimilars to Eylea (Regeneron Pharmaceuticals).

In an annual report, the company revealed the impact of a dramatic increase in commercially available biosimilars and progress in the areas of patient accessibility and treatment affordability.

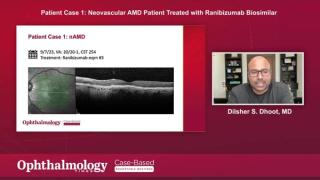
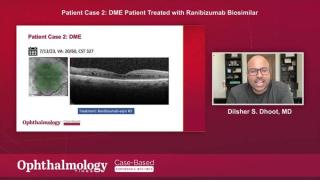
Two retina specialists participated in an Ophthalmology Times case-based roundtable discussion and shared their experiences using the anti-VEGF biosimilars, ranibizumab-eqrn and ranibizumab-nuna to manage retinal diseases.

According to the companies, the deal also includes Coherus’ CIMERLI biologics license application, ophthalmology sales and select field reimbursement teams. Coherus will now focus on its oncology business.

This study compared AVT06 with aflibercept (Eylea) in patients with neovascular AMD.

The ranibizumab biosimilar is a recombinant antigen-binding fragment (Fab).


ABP 938 is an investigational biosimilar to EYLEA® (aflibercept).