
According to Apellis Pharmaceuticals, the submission will be a Major Amendment to the NDA, extending the review period by three months with an expected PDUFA target action date in February.

According to Apellis Pharmaceuticals, the submission will be a Major Amendment to the NDA, extending the review period by three months with an expected PDUFA target action date in February.

Medicare’s 2023 fee schedule includes cuts in reimbursement, which some groups say could lead to reduced access to care to patients who need it most.

RGX-314 continues to be well tolerated in 50 patients from Cohorts 1-3 with no drug-related serious adverse events. The Phase II trial will be expanded to include higher third dose level, with patients stratified by DRSS levels across cohorts and all receiving short-course prophylactic ocular steroids following RGX-314 administration.

New details define the molecular patterns in thyroid eye disease and further implicate the role of insulin-like growth factor-1 in patients with low Clinical Activity Scores.

Ferdinand-Braun-Institute in Berlin, Germany is developing a solution with its semiconductor-based laser sources.

The novel genetic engineering approach, tested in mice and laboratory-grown nerve and light-receiving cells, will initially have research applications.
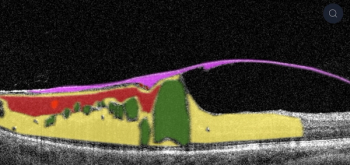
The company's AI cloud-based platform is available via a browser to any eye care professional in the world. The artificial intelligence algorithm was trained on millions of graphically labeled OCT scans collected from ophthalmic clinics.

According to National Institutes of Health/National Eye Institute researchers, the study may help lead to gene therapy for rare inherited blinding disease.

The study is one of the first clinical trials to validate the clinical utility of real-world remote physiologic monitoring using an on-demand vision-as-a-service extension to the specialty retina clinic to enable remote monitoring of patients with chronic diseases such as AMD, diabetic retinopathy, and other retinal conditions.
Nicox reports that daily dosing of NCX 470 0.1% met the primary efficacy objective of demonstrating non-inferiority to latanoprost 0.005%.

Replay is launching Eudora, an HSV gene therapy company that will target genetic retinal diseases.

Prevent Blindness offers videos, fact sheets, social media graphics and PowerPoint presentations to help ophthalmologists educate the public on the potential effects diabetes may have on vision.

Scott Walter, MD, an investigator in the TENAYA and LUCERNE trials discusses the year 2 data for the treat-and-extend regimen of faricimab for the treatment of nAMD and DME.

The EyeBuddy app will bring telehealth to eye doctors in the coming months, connecting patients across Bangladesh with ophthalmologists at a major eye-care hospital.

The FDA has set a PDUFA goal date of August 29, 2023. Bevacizumab-vikg, if approved, is expected to receive 12 years of regulatory exclusivity in the United States.
Preliminary data from the three-year, international study demonstrate safety and efficacy of NVK002 as a potential treatment for the progression of myopia in children.
Lloyd Paul Aiello, MD, moderated a panel at the American Academy of Ophthalmology’s 2022 annual meeting in Chicago that focused on diabetic retinopathy. He shares some of the highlights of this session.

Genentech announced this week that faricimab-svoa (Vabysmo) achieved its primary endpoint of non-inferiority compared to aflibercept in RVO in the BALATON and COMINO clinical trials.
A previously uncharacterized area of the protein known as RPE65 spontaneously turns spiral-shaped when it encounters intracellular membranes, or thin structures that surround different parts of a cell.

Marjan Farid, MD, discussed the latest updates in femtosecond laser keratoplasty during a presentation at the American Academy of Ophthalmology’s 2022 annual meeting in Chicago. She shared the highlights of that presentation here.

Arun Singh, MD, discusses a presentation on iris lesions he gave recently at the American Academy of Ophthalmology’s 2022 annual meeting in Chicago on the use of small incision garden hydroaspiration of iris lesions.

The 10-year retrospective ALOFT study details the importance of digital remote monitoring for patients diagnosed with age-related macular degeneration.

Teprotumumab-trbw is the first and only medicine approved by the FDA for the treatment of thyroid eye disease.

Harit Bhatt, MD, a Chicagoland-area retinal surgeon, shared his experience with the LEAF and LION portable lasers during a conversation at the American Academy of Ophthalmology’s 2022 annual meeting in Chicago.

Vivek Patel, MD, discusses issues that could threaten sight during presentation at the American Academy of Ophthalmology annual meeting in Chicago.

Ula V. Jurkunas, MD, discusses a presentation she gave recently at the American Academy of Ophthalmology’s 2022 annual meeting in Chicago on the use of limbal stem cells to treat corneal blindness.

The company noted that the pediatric exclusivity determination is based on data from two Phase 3 trials – BUTTERFLEYE and FIREFLEYE.

Michelle Senchyna, PhD, provided an update of the COMET program and other development activities at Aerie Pharmaceuticals during a conversation at the American Academy of Ophthalmology’s 2022 annual meeting in Chicago.

During a conversation at the American Academy of Ophthalmology’s 2022 annual meeting in Chicago, Rishi Singh, MD, offered highlights of the two-year results of faricimab in a pair of phase 3 clinical trials.

According to NEXGEL, the patch is expected be available for ophthalmologists to offer to their patients in the first half of 2023.