Ocular Allergy
Latest News
Latest Videos

CME Content
More News
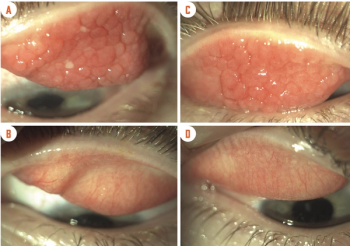
Most patients with allergic disorder are children, adolescents, or young adults.
Santen Inc. announced Thursday that the U.S. FDA has approved Verkazia (cyclosporine ophthalmic emulsion) 0.1% eye drops to treat vernal keratoconjunctivitis (VKC).

According to investigators, the system has been shown to almost triple the number of people with eye problems attending primary care, as well as increasing appropriate uptake of hospital services.

The event, being held at the Mandalay Bay Resort and Casino in Las Vegas, is offering a full agenda for in-person attendees.

EyeGate Pharmaceuticals, Inc.'s lead product candidate is designed to treat dry eye disease-induced ocular surface inflammation.
Delivering existing therapeutics through alternative routes may offer option.

Autologous serum eye drops and blood products offer hope to patients.
Growing patient pool is driving clinical trial investment.

Ocular symptoms in COVID-19 patients may be more common than previously thought — with sore eyes a significant sign of disease.
First new therapy in nearly a decade offers another option for physicians, patients
Study and use of platelet preparation, other biologics viewed as next frontier

Hydrogel technology enables the use of drugs that are known to be efficacious for ocular diseases and conditions when formulated as daily drops or monthly injections, into one-time or several-month dosage forms.

Half of patients with ocular allergies report experiencing symptoms year-round. While nearly all of them take eye drops to treat their symptoms, the majority report limited or no effect from over-the-counter drops, according to a new survey. The results suggest that new treatment approaches would improve both symptoms and quality of life.

Oral cetirizine is one of the most used oral medications for treatment of allergic rhinitis. In May 2017, the FDA approved the first ophthalmic formulation of the second-generation histamine-1 (H1) receptor antagonist for use in treating ocular itching associated with allergic conjunctivitis.

Progress of ocular allergy treatment in 2017 starts with the fact that this is a mature therapeutic space, with a range of existing choices for clinicians and patients. Progression in therapies from artificial tears to antihistamines and mast cell stabilizers to topical steroids provides a suitable choice for most patients with ocular allergies.

A phase III study of dexamethasone insert, 0.4 mg (Dextenza, Ocular Therapeutix) found that the sustained-release intracanalicular insert is safe, effective, and well tolerated for treating ocular itching associated with allergic conjunctivitis.

Isunakinra, an interleukin-1 signaling inhibitor designed for topical ophthalmic administration, did not meet the primary endpoint in a phase III clinical trial for the treatment of moderate-to-severe allergic conjunctivitis.

Alcaftadine ophthalmic solution 0.25% was superior to olopatadine hydrochloride ophthalmic solution 0.2% in reducing the itching related to grass and tree pollens in a multicenter, randomized study in subjects with allergic conjunctivitis.

A new, first-in-class, aldehyde-trap topical drop demonstrated rapid onset of action and sustained efficacy with an acceptable safety profile in a phase II clinical trial of patients with moderate-to-severe allergic conjunctivitis.

The arrival of spring also signals the onset of ocular allergy season. Clinicians can prepare by knowing and recognizing the symptoms in their patients, as well as being informed about the latest therapies for this diagnosis.

Being armed with as much information as possible about a patient’s ocular allergies can make a major difference in treatment decisions, whether for dry eye or scheduling of ocular surgery.

Treatment of chronic allergic conjunctivitis with a sustained-release dexamethasone resulted in better clinical results because of the sustained and controlled delivery.

Ocular surface disease may be multifactorial, presenting a challenge to the physician in making a correct diagnosis and management plan.

Olopatadine 0.2% is a well-tolerated, safe, and effective antihistamine/mast cell stabilizer for treating mild to severe allergic conjunctivitis in children and adults.

A novel dexamethasone-eluting punctum plug was safe and showed some evidence of efficacy for relieving signs and symptoms of allergic conjunctivitis in a phase II study.