Retina
Latest News
Latest Videos

Shorts




CME Content
More News


Analysis of 20 years of data highlights differences in reimbursement trends across ophthalmology subspecialties and practice settings, according to Dustin D. French, PhD.

The trial is evaluating OCU410 (AAV5-RORA) for the treatment of geographic atrophy (GA) secondary to dry age-related macular degeneration.

OCU410ST uses an adeno-associated virus delivery platform for the retinal delivery of the RORA gene.

The Part B dose-expansion portion is evaluating SB-007 for the treatment of Stargardt disease.

Optoretinography is an emerging technology used to test light-evoked photoreceptor activity.

Their conversation focuses on anatomy-driven, individualized approaches and multidisciplinary decision-making for patients with neovascular glaucoma.

Researchers uncover a DNA repair mechanism in Greenland sharks that preserves their vision for centuries, offering insights into longevity and eye health.

According to the company, the FDA’s decision was supported by visual function results from the phase 2 ACUITY trial in ON.
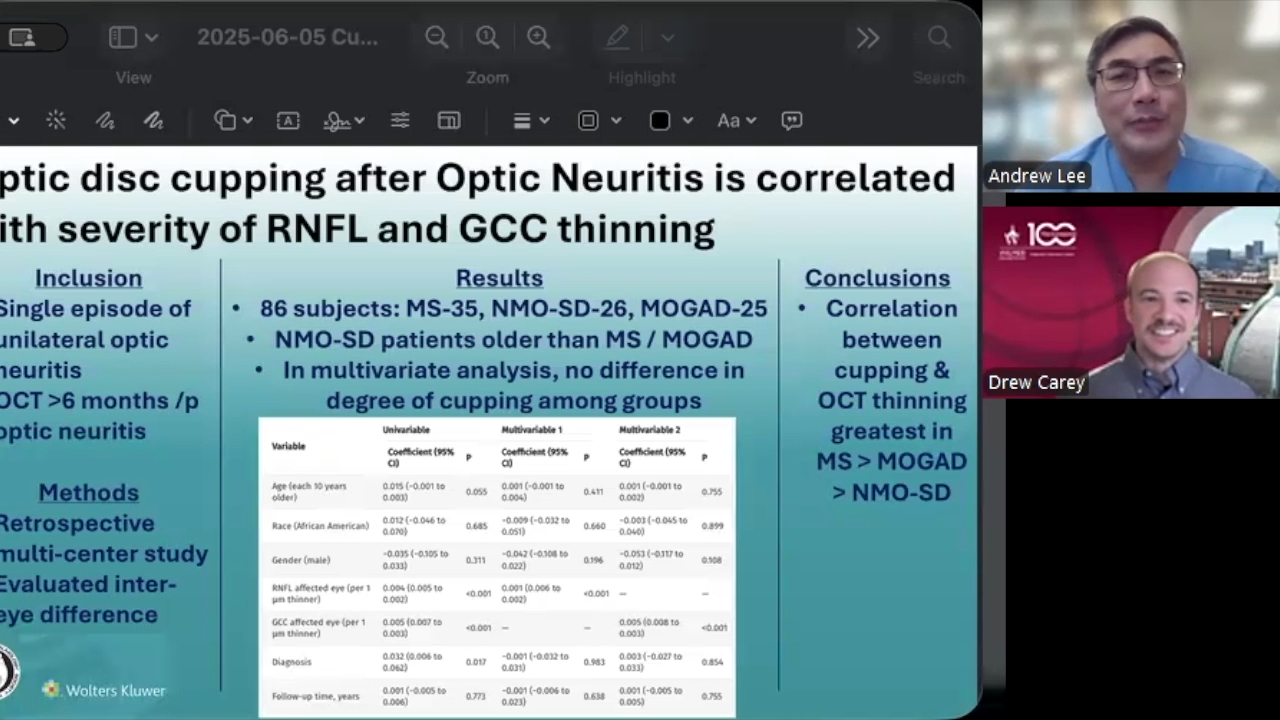
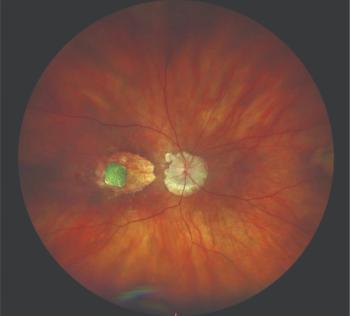
A recent study represents a step forward in investigations of the retinal layers as they are affected by glaucoma.

Jennifer E. Thorne, MD, PhD, discusses evidence from the ADJUST trial, including relapse risk, retreatment success, and how clinicians should monitor children when considering adalimumab discontinuation.

The letter noted the FDA is unable to approve the application for ONS-5010/LYTENAVA (bevacizumab-vikg) in its current form for the treatment of wet AMD, according to the company.

An in-depth look at the FDA approvals and clinical milestones that defined 2025 and how they are poised to influence practice patterns moving into 2026.
The novel system converts light into electrical signals to stimulate retinal cells.
Strategies for treatment intervals, drug selection, and patient expectations.

A trio of retina specialists recently reviewed the clinical benefits of aflibercept 8 mg, including its extended dosing intervals, improved patient satisfaction, and enhanced treatment outcomes for various conditions.



Leading cataract surgeons discuss their comprehensive approach to IOL counseling, exploring how they bridge expectation gaps and tailor premium lens strategies to each patient's unique anatomy, goals, and lifestyle.

The DRAGON trial was a 24-month pivotal phase 3 trial evaluating Tinlarebant in adolescent STGD1 patients.

Kiora Pharmaceuticals advances ocular therapies with new patents for KIO-104, targeting retinal inflammation and enhancing treatment options.

Both therapeutics will leverage AGC Biologics’ BravoAAV suspension platform and use an innovative dual AAV vector approach, which splits the therapeutic gene into 2 halves.