
Vizz (Lenz Therapeutics) is the first and only aceclidine-based eye drop for presbyopia and is also the first daily solution to correct vision for up to 10 hours.

Vizz (Lenz Therapeutics) is the first and only aceclidine-based eye drop for presbyopia and is also the first daily solution to correct vision for up to 10 hours.
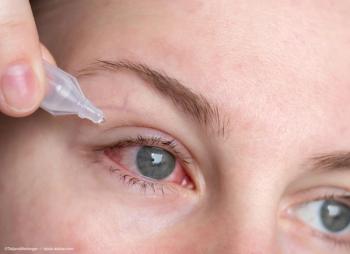
In addition to Prevent Blindness' usual free resources, the organization is providing a dedicated web page, fact sheets, and graphics in English and Spanish.
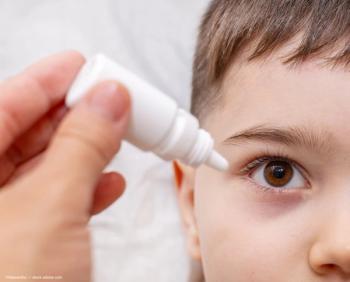
IVMED-85 is a preservative-free, non-atropine daily drop that slows myopia progression by strengthening scleral and corneal collagen crosslinks via LOX activation.

The FDA has set a Prescription Drug User Fee Act (PDUFA) date of January 28, 2026.
Deep Parikh, MD, sat down with Emily Kaiser Maharjan from Optometry Times to talk about his presentation at Collaborative Care Symposium on advanced treatments in neovascular retinal disease.

Alcon’s dry eye candidate acoltremon 0.003% is a first-in-class thermoreceptor agonist, which stimulates corneal sensory nerves to increase natural tear production to treat the signs and symptoms of dry eye disease.

Following his keynote address at Controversies in Modern Eye Care, Dr. Maloney said he will be leaving his surgical practice and returning to research.

In this Q&A, John D. Gelles, OD, and Steven Greenstein, MD, chat about what inspired them to launch the symposium, what makes this year’s agenda especially exciting, and why the future of refractive care depends on stronger collaboration between ODs and MDs.
Steven Greenstein, MD, one of the cochairs of the Collaborative Care Symposium gave some insight into the upcoming conference and what attendees can expect.

Susvimo is the first and only FDA-approved, continuous delivery treatment shown to maintain vision in people with DR with just one refill every 9 months




In this Q&A, Luke Lindsell, OD, MD, shares his insights on emerging therapies in retina, the evolving role of optometrists in retinal disease management, and the importance of comanagement in patient care.


Wendy Lee, MD, MS, shares how she integrates aesthetic and functional care in oculoplastics.
ENTOD Pharmaceuticals launches Myatro XL, a 0.05% atropine eye drop, to effectively slow myopia progression in children, available by prescription in 2025.

Under the Vevye Access for All program, patients with a Klarity-C prescription can switch to Vevye for $59 per bottle.

This program is in place for prescriptions of cyclosporine ophthalmic solution 0.1% (VEVYE, Harrow) and promises a $59 price tag per bottle, effective immediately.

The FDA has set a PDUFA target action date of October 23, 2025, for the low-dose atropine formulation.

A PDUFA date has been set for October 20, 2025. If approved, Epoxia will be the first epi-on corneal crosslinking on the market.
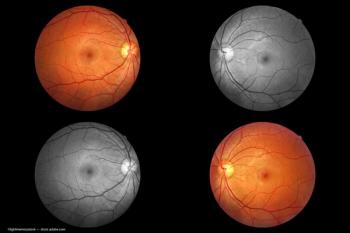
MonacoPro, the next evolution of Monaco from Optos, retains the powerful ultra-widefield SLO and spectral domain imaging while adding additional key product features.

The approval follows a refiling after the FDA issued a Complete Response Letter due to language on the amended label.

EssilorLuxottica’s Nuance Audio glasses, which are equipped with hearing aids, will be available over the counter in the US and Europe as soon as Q1 2025.

With this addition, Zeiss completes the Corneal Refractive Workflow, which already includes Visumax 800 and SMILE pro.

This year was brimming with advancements in eye care.
A PDUFA decision date of April 2, 2025 has been assigned

At the Tear Film and Ocular Surface meeting in Venice, Melissa Toyos shares findings on the mechanism of action of brimonidine tartrate.

New research sheds light on how the dry eye medication lifitegrast works beyond the ocular surface, targeting the underlying immune system to provide relief for patients.

Published: March 11th 2025 | Updated:

Published: October 3rd 2022 | Updated:

Published: February 28th 2022 | Updated:

Published: October 30th 2022 | Updated:

Published: May 12th 2022 | Updated:

Published: September 22nd 2022 | Updated: