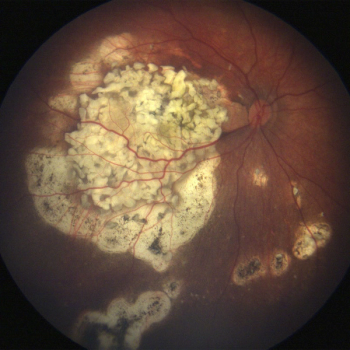
Children who develop the pediatric eye cancer retinoblastoma often lose their vision or an eye due to a lack of specific, targeted therapies and a poor molecular understanding of the cancer. Researchers at UT Southwestern and the University of Miami have discovered that a molecule – estrogen-related receptor gamma, or ESRRG – becomes hyperactive and promotes tumor cell survival in retinoblastoma.