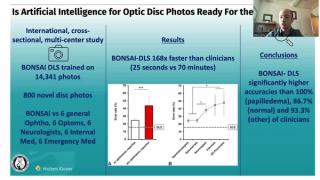
Clinical Diagnosis
Latest News

Study: Prevention of age-related truncation of γ-glutamylcysteine ligase catalytic subunit delays cataract formation
Latest Videos

CME Content
More News

Alice Epitropoulos, MD, discusses the importance of considering ocular surface health in cataract surgery patients.
Ophthalmologist shares pearls from recent roundtable discussion on topic.

Physicians note the impact of prism lenses can be life-changing for patients.
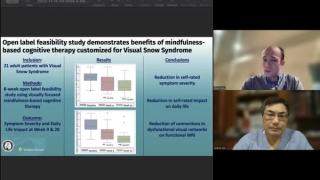
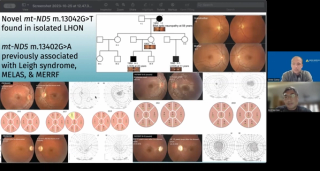
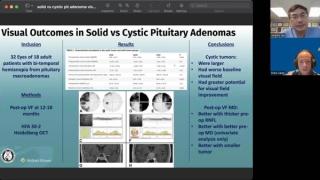
Research can drive opportunities for patients with rare diseases
Slit lamp evaluation can provide valuable information for treatment course.

Ophthalmic data are well suited to the demands of regulatory-grade use cases.

Resolution of macular leakage is an early biomarker of vascular stability.

Leaders must confront issues perpetuating unequal access, unfair practices.

In this podcast episode, Ophthalmology Times' co-chief medical editors Peter J. McDonnell, MD, and Neda Shamie, MD, delve into the clinical nuances of Demodex blepharitis. Through personal anecdotes, they stress its underrecognized gravity and discuss diagnostic cues like collarettes. The experts share their enthusiasm for improved treatments, echoing the transformative impact seen in dry eye care.

Andrew Lee, MD, Andrew Carey, MD, and Elizabeth Fortin, MD, sit down on this episode of the NeuroOp Guru to discuss neuro-ophthalmology, interprofessional eConsults, and whether they provide timely and valuable guidance

Condition is linked to longer resolution times, worse outcomes

Surgeon discusses latest trends for treating cornea.
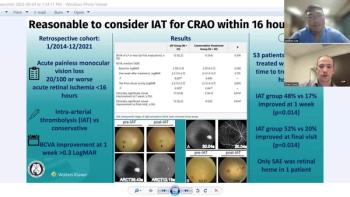
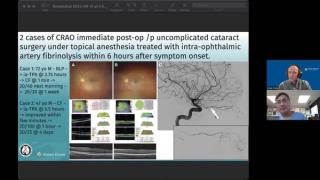
Andrew Lee, MD, and Andrew Carey, MD, sit down on another episode of the NeuroOp Guru to discuss IAT for CRAO within 16 hours.

According to Horizon Therapeutics, the analysis shows subclinical spinal cord lesions were associated with future NMOSD attacks. The analysis was being presented at the 9th Congress of the European Academy of Neurology in Budapest.

William B. Trattler, MD, highlights some of the novel therapies for dry eye that have recently been approved by the FDA, along with various treatment approaches that are in development.

On this episode of the NeuroOp Guru video blog, Andy Lee, MD, and Drew Carey, MD, discuss the use of fluoxetine in patients with visual field loss after suffering a stroke.

Dual therapy may slow the progression of condition.

Medical, surgical management can be important tools.

John D. Gelles, OD, and Steven A. Greenstein, MD, share some insights into the new data on corneal cross-linking presented at IKA Keratoconus Symposium.

At the IKA Keratoconus Symposium, Justin Schweitzer, OD, FAAO, presented on establishing the keratoconus diagnosis alongside Kourtney Houser, MD.

President and CEO of the International Keratoconus Academy Andrew Morgenstern, OD, FAAO, FNAP, shares some snippets about the live white paper development session and the patient Q&A happening at the inaugural IKA Keratoconus Symposium.

Uri Soiberman, MD, shares some key takeaways from his presentation at the inaugural IKA Keratoconus Symposium.

A successful career in ophthalmology may seem daunting to a young trainee, but Real World Ophthalmology is here to reveal the secrets.

Cochair Elizabeth Yeu, MD, highlights what this program will mean for ophthalmologists, optometrists, and their patients.
Lisa M. Nijm, MD, MD, highlights what residents, fellows, and ophthalmologists who are early in their careers can expect at the virtual Real World Ophthalmology meeting on Saturday, April 15.