Clinical Diagnosis
Latest News
Prevent Blindness has designated November 18 to 24, 2024, as the 5th annual Thyroid Eye Disease (TED) Awareness Week. The week aims to raise awareness of TED's symptoms, impact on vision and mental health, and new treatment options. Educational resources and expert-led episodes will support patients and healthcare professionals.

Study examines the use of AI–based detection of diabetic retinopathy in the US

Noninvasive choroidal vessel analysis using deep learning: A novel approach to OCT angiography
Latest Videos

CME Content
More News
A study led by researchers at the University of Birmingham finds AI-powered models can match ophthalmologists in diagnosing infectious keratitis, offering promise for global eye care improvements.

A recent paper reveals increasing rates of diabetic retinopathy in young patients with diabetes, sparking concerns over inadequate screening and management, especially among Black and Hispanic youths.
A team of researchers at Arizona State University has developed tools to aid in the diagnosis of myopic maculopathy.

Lotfi Merabet, OD, PhD, MPH, discusses his role as co-chairman of a National Eye Institute initiative focused on advancing research in cerebral visual impairment (CVI). The initiative's key goals include raising awareness about CVI, establishing clear diagnostic criteria, and developing a national registry to track patients and their conditions over time. CVI, a complex condition that affects how the brain processes visual information, requires a multidisciplinary approach involving not only ophthalmologists and optometrists but also neuroscientists, educators, and rehabilitation specialists.
Adam S. Wenick, MD, PhD, discussed myopia interventions across the lifespan at EyeCon 2024, covering treatments, complications, and prevention strategies.
Adam S. Wenick, MD, PhD, chaired a symposium on geographic atrophy (GA) at EyeCon 2024, focusing on the diagnosis, monitoring, and FDA-approved treatments for GA. The session with Leonard Messner, OD, FAAO, also included case presentations that highlighted key factors for clinicians to consider when selecting appropriate treatment options.

Ehsan Sadri, MD, FACS, of Visionary Eye Institute in Newport Beach, California, sat down with Professor Emeritus Colin R. Green, PhD, DSc, University of Auckland, in Auckland, New Zealand, to learn more about a novel therapy in development for the treatment of keratoconus and other corneal ectasias.

Laura M. Periman, MD, shares her passion for dry eye disease, discussing her discovery of the Alpenglow Sign in Demodex blepharitis and her insights on research and education for clinicians. Periman will join the EyeCon 2024 faculty as Honorary Chair.
David A. Eichenbaum, MD, presented key data from two clinical trials focusing on therapies for age-related macular degeneration at the Retina Society 57th Annual Scientific Meeting in Lisbon, Portugal.

Push to identify biomarkers for earlier diagnosis, new therapeutic targets.
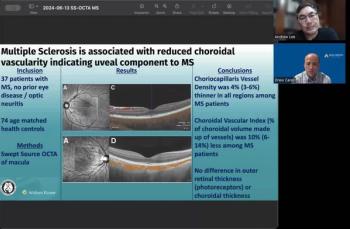
Andrew Lee, MD, and Andrew Carey, MD, sit down on another episode of the NeuroOp Guru to discuss Multiple Sclerosis and its association with reduced choroidal vascularity.

In this PER On Air Ophthalmology Times podcast, an expert panel discusses the latest advances in X-linked retinitis pigmentosa and how to optimize diagnosis as well as developments in gene therapy.
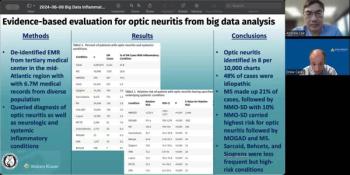
Andrew Lee, MD, and Andrew Carey, MD, sit down on another episode of the NeuroOp Guru to discuss evidence-based evaluation for optic neuritis from big data analysis

In this PER On Air Ophthalmology Times podcast, an expert panel discusses diagnosing TED and providing appropriate pharmacologic and surgical treatments.

Current Fuchs endothelial corneal dystrophy treatments include implanted cultured human endothelial cells.
Cochair Kelly K. Nichols, OD, PhD, MPH, FAAO, highlights her passion for dry eye research and the vital collaboration between ophthalmology and optometry at EyeCon.
Early diagnosis of NMOSD is key to preventing disability, improving survival.

Researchers train deep learning model on OCT scans to ID patients eligible for GA trials.
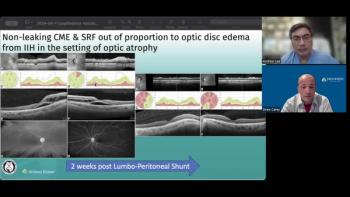
Andrew Lee, MD, and Andrew Carey, MD, sit down on another episode of the NeuroOp Guru to discuss nonleaking cystoid macular edema and subretinal fluid out of proportion to optic disc edema in idiopathic intracranial hypertension in the setting of optic atrophy.
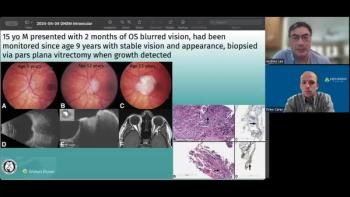
Andrew Lee, MD, and Andrew Carey, MD, sit down on another episode of the NeuroOp Guru to discuss a unique case of a 15-year-old who showed signs of meningioma with epithelial whirls and some psammoma bodies in the eye.

According to Genentech, faricimab-svoa is the first and only syringe prefilled with an FDA-approved bispecific antibody to treat retinal conditions that can cause blindness.

In this PER On Air Ophthalmology Times podcast, a team of experts provides insights into the diagnosis and management of patients with NK (including assessing their underlying cause of NK), with a look at the range of treatments available for patients with advanced stages of NK.

This PER On Air - Ophthalmology Times podcast examines the new treatment paradigm for patients with geographic atropy, following the introduction of complement-targeting therapies.

In this Ophthalmology Times EyePod podcast, Mandeep S. Singh, MD, PhD, speaks with Mark S. Humayun, MD, PhD, about the progress made by a team of USC researchers on a novel stem cell patch for treating advanced dry age-related macular degeneration.
Optimizing corneal neurotization outcomes with cryopreserved amniotic membrane.