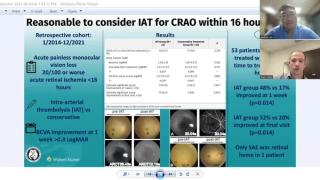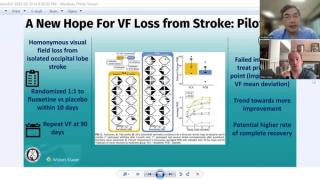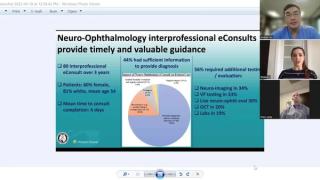
Clinical Diagnosis
Latest News

Latest Videos

CME Content
More News

Cochair Elizabeth Yeu, MD, highlights what this program will mean for ophthalmologists, optometrists, and their patients.
Lisa M. Nijm, MD, MD, highlights what residents, fellows, and ophthalmologists who are early in their careers can expect at the virtual Real World Ophthalmology meeting on Saturday, April 15.

The month will serve to educate the public on a variety of women’s vision issues.

Disruption affected ophthalmology, altering physicians’ education, careers.

Many patients with diabetes can be stabilized with anti-VEGF biologics.

Ehsan Sadri, MD, FACS, and William Trattler, MD, highlight some of the pending FDA approvals and PDUFA dates in the anterior segment sector and what these products will mean for ophthalmologists and their patients.

Ophthalmologist looks ahead amid company’s advances over past 18 months.

It is important for patients to understand what digital eye strain is and its causes.

Long-term screen users and contact lens wearers in group face challenges.

This subtype of artificial intelligence technology may have long-range implications for clinical trials.

The considerations for biosimilars in clinical practice are interesting and remain to be determined, according to George A. Williams, MD.
The survey addressed topics that would facilitate a better understanding of the awareness of retina specialists regarding this relative new form of treatment in ophthalmology.
Bonnie An Henderson, MD, founder and program director of EnVision Summit, previews what attendees can expect at this year’s meeting - from its diverse faculty to an inclusive Youth Program, while also giving back to the community. The conference will take place February 17 to 20 at the El Conquistador Resort in Fajardo, Puerto Rico.
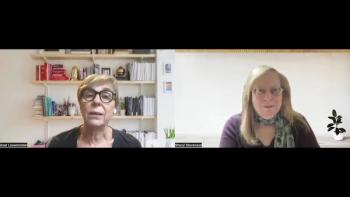
The approval of aflibercept for preterm infants with retinopathy of prematurity (ROP) expands the treatment armamentarium for these patients, comments Prof Anat Loewenstein.

Robert L. Stamper, MD, discusses some of the devices in development for measuring patients' intraocular pressure outside of doctors' office hours in his presentation at Glaucoma Research Foundation's annual Glaucoma Symposium.
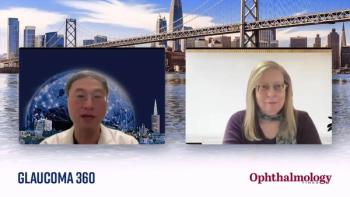
Shan C. Lin, MD, provides an overview of the various intraocular lens technologies that are available, as well as considerations for contrast sensitivity and vision loss in patients at Glaucoma Research Foundation's annual Glaucoma Symposium.

Kristen H. Ingenito, MBA, vice president and director of ophthalmics at Market Scope, discusses the volume of ophthalmologists offering interventional glaucoma treatment as well as emerging therapies in her presentation at the Glaucoma 360 New Horizons Forum.

Let us know how familiar are you on the approval process for biosimilar drugs compared with new drug applications to the FDA. The poll will be open until February 28, 2023.
Timely access to effective diagnosis and cross-linking is key to positive outcomes

Continued education and financial incentives will become essential if biosimilars are to become a mainstay in ophthalmological markets, according to a recent report.

Automated AI tool offers offline capability and hope for patients.

Previous research conducted primarily by scientists at the University of São Paulo, Brazil, showed brilacidin can potentiate several marketed antifungals, including caspofungin, voriconazole and posaconazole.

Survey of academic chairs and residency directors recognizes centers of excellence in Best Clinical Care, Best Residency, Best Research, and Best Overall Programs.

Spanish investigators found that local resection may offer better visual results and eye sparing without compromising local tumor control and survival.
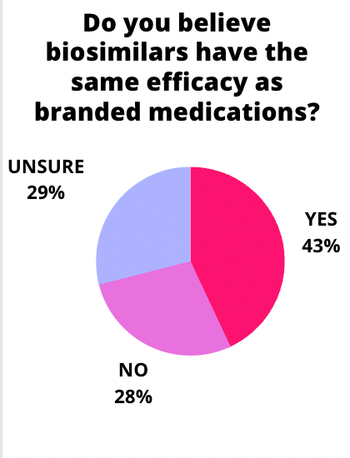
Results from our recent poll indicate a mixed bag of responses from ophthalmologists.