Clinical Diagnosis
Latest News

Clearside Biomedical retinal therapeutic meets primary endpoint in OASIS phase 1/2a trial

Viatris making ophthalmology push with acquisition of Oyster Point Pharma
Latest Videos

CME Content
More News

New details define the molecular patterns in thyroid eye disease and further implicate the role of insulin-like growth factor-1 in patients with low Clinical Activity Scores.

Jay S. Duker, MD, chief operating officer of EyePoint Pharmaceuticals, highlights the Phase 1 and Phase 2 DAVIO clinical trials for EYP-1901 in wet age-related macular degeneration. The company also announced the first patient has been dosed in the Phase 2 PAVIA trial of EYP-1901 for nonproliferative diabetic retinopathy.

Nicole Bajic, MD, and Neel S. Vaidya, MD, MPH, share their insights from when they were at this crossroads. Learn more clinical pearls on topics like this and more at Real World Ophthalmology’s virtual conference, “Top 10 Things I Wish I Knew Sooner,” this Saturday, November 5.

The study is one of the first clinical trials to validate the clinical utility of real-world remote physiologic monitoring using an on-demand vision-as-a-service extension to the specialty retina clinic to enable remote monitoring of patients with chronic diseases such as AMD, diabetic retinopathy, and other retinal conditions.

Lawrence Whittle, Chief Commercial Officer for Verana Health, explains what the significance of imaging data linked with electronic health record data means for patient outcomes.
Nicox reports that daily dosing of NCX 470 0.1% met the primary efficacy objective of demonstrating non-inferiority to latanoprost 0.005%.

Replay is launching Eudora, an HSV gene therapy company that will target genetic retinal diseases.

Prevent Blindness offers videos, fact sheets, social media graphics and PowerPoint presentations to help ophthalmologists educate the public on the potential effects diabetes may have on vision.

Scott Walter, MD, an investigator in the TENAYA and LUCERNE trials discusses the year 2 data for the treat-and-extend regimen of faricimab for the treatment of nAMD and DME.

The EyeBuddy app will bring telehealth to eye doctors in the coming months, connecting patients across Bangladesh with ophthalmologists at a major eye-care hospital.

Marc Berger, Chief Operating Officer at Verana Health, highlights what a new initiative with the American Academy of Ophthalmology IRIS Registry will mean for patients and physicians.
Complimentary registration is open for this unique meeting designed to prepare young ophthalmologists for success in early practice.
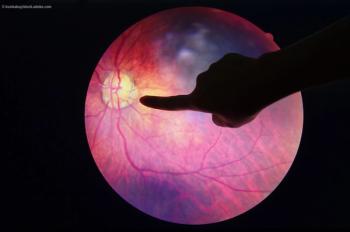
A team of investigators evaluated VA outcomes after cataract surgery and the factors associated with good visual outcomes in a population of patients diagnosed with type 2 diabetes.

There literally are not enough hours in a day to allow physicians to comply with all the recommendations emanating from the various constituencies involved in issuing guidelines.

Ula V. Jurkunas, MD, discusses a presentation she gave recently at the American Academy of Ophthalmology’s 2022 annual meeting in Chicago on the use of limbal stem cells to treat corneal blindness.
The COVID-19 pandemic has led to greater comfort with robotic telepresence in eye screenings.

The company noted that the pediatric exclusivity determination is based on data from two Phase 3 trials – BUTTERFLEYE and FIREFLEYE.

An interactive agenda at the Ophthalmology Times® EyeCon 2022 at the JW Marriott Marco Island Beach Resort in Florida will foster an open exchange of ideas with fellow attendees from across the United States.

According to Penny Asbell, MD, FACS, MBA, the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) surveillance study has identified high levels of in vitro antibiotic resistance among ocular staphylococcal and pneumococcal pathogens, resistance that may affect treatment success. She discussed the program at the American Academy of Ophthalmology’s 2022 annual meeting in Chicago.

Lisa M. Nijm, MD, JD, shares highlights from the Real World Ophthalmology 'After Dark' networking reception held at the American Academy of Ophthalmology annual meeting. The next Real World Ophthalmology meeting on November 5—which will be virtual—will cover important clinical, business, and personal growth areas for young ophthalmologists.

According to a BBC News report, the woman showered while wearing her lenses and she contracted a rare parasite infection called Acanthamoeba keratitis.
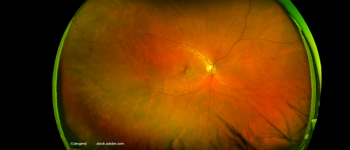
Baseline age, best-corrected visual acuity, and central subfield thickness play role in predicting the efficacy of both drugs.
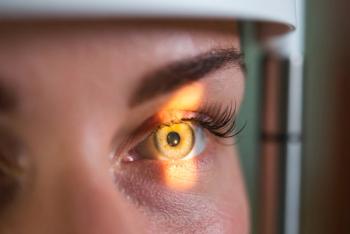
According to Thomas Aaberg, MR, the novel therapy is geared to treat rare, debilitating eye disease.
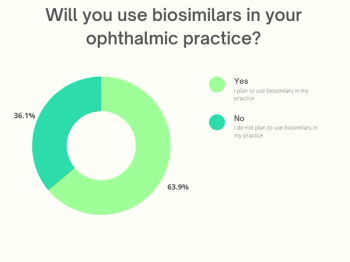
Results from our recent poll indicate that many ophthalmologists (63%) plan to use biosimilars in their clinical practice.
Anterior segment surgeon expands on the use of the XtraFocus pinhole implant.