Articles by OT Staff Reports
Ocular herpes infection is a leading cause of infectious blindness and can lead to lethal brain infections. Using genetically modified mice models, investigators uncovered the importance of mTORC2 in activation of innate- and virus-adaptive immunity during ocular HSV-1 infection.

In honor of World Sight Day, staff from our parent company, MJH Life Sciences, share their perspectives on the significance of vision in their lives.
Oxurion NV announced Wednesday the first patient to be dosed in the INTEGRAL phase 2 clinical study evaluating THR-687 as a treatment for patients with DME.

Aerie Pharmaceuticals announced Tuesday positive topline results for the phase 3 trial evaluating netarsudil 0.02% versus ripasudil 0.4% for the treatment of open-angle glaucoma and elevated IOP.

The FDA approved Ocular Therapeutix Inc.’s Supplemental New Drug Application (sNDA) that further extends Dextenza’s (dexamethasone ophthalmic insert) 0.4 mg indications, the company announced Monday.

Justis P. Ehlers, MD, speaks on the findings from a higher-order OCT analysis for inflammatory signal biomarkers in the HAWK Study.

Alcon's Paul Hallen offers a look at the highlights from the 2021 ASRS annual meeting's OIS panel as well as the company's latest innovations.

Caroline Baumal, MD, highlights results from the Phase 3 YOSEMITE and RHINE trials, providing an overview of the efficacy, safety and durability of faricimab in DME.

According to 4D Molecular Therapeutics, it has received FDA Clearance of an IND Application for 4D-150, a dual-transgene intravitreal gene therapy for patients with wet AMD.
The company will move the highest dose of THR-149 (0.13mg) into Part B of the study based on favorable safety profile and positive efficacy data.
iCare USA’s EIDON Ultra-Widefield Lens module has received FDA 510 (k) approval for distribution across the United States, the company announced Wednesday.
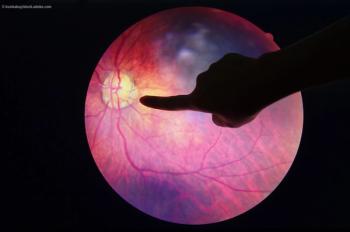
Investigators found evidence of improvement in vision and retinal structure in patients with DME and AMD sustained through 12 weeks.

A team of investigators at the Texas Tech University Health Sciences Center have developed a technology derived from corneal epithelial stem cells to improve outcomes for DED patients.

According to researchers at the University of Virginia School of Medicine, common HIV drugs or a safer alternative could stop vision loss.
Graybug Vision reported Tuesday a full-data analysis from the phase 2b ALTISSIMO trial of GB-102 for the treatment of wet AMD.

The screening device works by assessing the eyes’ ability to fixate together. Held 14 inches from the eyes, the child fixates on a smiley face while the device simultaneously scans both retinas.

According to a survey commissioned by Allergan, an AbbVie company, 65% of respondents said they were not prepared to have their vision worsen as they aged.
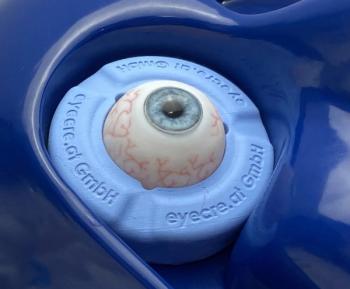
Addion GmbH has selected Stratasys technology to create realistic eye models for ophthalmic surgery.

American Society of Retinal Specialists program to drive awareness and action around retinal diseases that put millions at risk for blindness.

The company announced that inebilizumab-cdon produces rapid and sustained B-cell depletion in African Americans with neuromyelitis optica spectrum disorder.
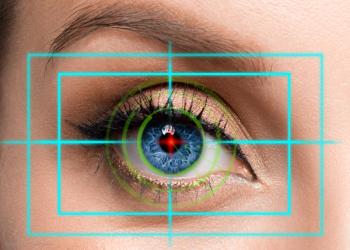
A recent report by Heru Inc. has found that visual field (VF) results from re:Vive, its wearable testing platform, have a strong association to the ZEISS Humphrey Field Analyzer (HFA).
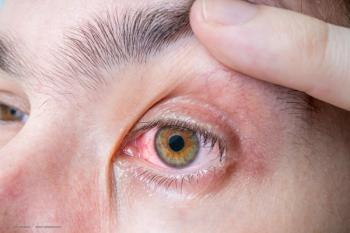
Glaukos has entered into a licensing agreement with Attillaps Holdings Inc., giving it an exclusive license to research, develop, manufacture and commercialize Attillaps’ proprietary library of investigational pharmaceutical compounds that target the eradication of Demodex mites.

The company will host Innovation Week from Sept. 21-23, demonstrating new data management innovations across devices, workflow solutions and software applications.

According to Samsung Bioepis and Biogen, ranibizumab-nuna becomes the first ophthalmology biosimilar to gain FDA approval in the United States.

Investigators are working on the development of an intranasal subunit vaccine that provides durable local immunity against inhaled pathogens.

The organization’s policies come amid federal mandates designed to spur Americans who are not vaccinated against COVID-19 to get a shot.

TearLab, MellingMedical reach an agreement to enhance early detection of dry eye disease in veterans.
A team of investigators have found that a molecular mechanism drives the progression of uveal melanoma, an often lethal eye cancer in adults.

Investigators at Dartmouth College have found that eye contact during a conversation makes the discussion more appealing to its participants.
Lisa Nijm, MD, JD, discusses the brand-new Real World Ophthalmology conference, designed to support ophthalmologists starting practice.






























