
At AAO, AbbVie presented results from the ARTEMIS study, showcasing IOP lowering that extends beyond the original 20-month timeframe.

At AAO, AbbVie presented results from the ARTEMIS study, showcasing IOP lowering that extends beyond the original 20-month timeframe.
Researchers have found that study participants with healthy eyes and no history of AMD had thinner retinas if they carried the genes that put them at risk.

For 72 straight hours, the study volunteer lay in a bed at UT Southwestern, the monotony broken only at night when researchers placed his lower body in a sealed, vacuum-equipped sleeping bag to pull down body fluids that naturally flowed into his head while supine.
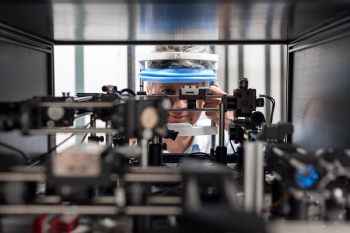
A team of scientists have developed a two-photon excited fluorescence scanning laser ophthalmoscope, an instrument that allows viewing the biochemistry of vision in the living eye in real time.

At the 2021 American Academy of Ophthalmology annual meeting, Penny Asbell, MD, FACS, MBA, discussed data from the ARMOR study and its effects on treating ophthalmic infections, such as endophthalmitis.

A team of researchers with the West Virginia School of Medicine are studying how a benign virus can make new treatments for eye diseases possible.

A team of researchers from Thomas Jefferson University have found that immune cells could be doing much more than first thought in protecting our eyes.
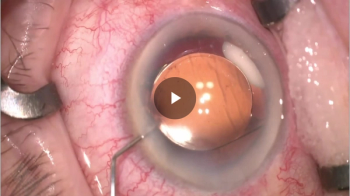
Robert H. Osher, MD, shows the merits of drug delivery via capsular bag after cataract surgery.

The cell-by-cell atlas will help in the study of eye disorders and development of cell therapy to replace damaged eye tissue.
Mahmoud A. Khaimi, MD, developed a minimally invasive glaucoma surgery known as ab-interno canaloplasty as a treatment option between topical therapies and invasive surgery. The technique continues to prove its value.

According to the company, a suprachoroidal injection of CLS-AX 0.1 mg dose was well-tolerated in cohort 2 with no treatment related adverse events, while the consistent safety profile observed in in cohorts 1 and 2 supports advancement to cohort 3.

Researchers found there was, on average, a 17% improvement in participants’ color contrast vision when exposed to three minutes of deep red light in the morning.

New cell therapy drug makes treatment of corneal endothelial disease more accessible.
The company continues to develop testing options that deliver accurate detection and correctly identify SARS-CoV-2 mutations to help patients and healthcare providers manage the evolving COVID-19 pandemic.

The company anticipates that the Sirius and Celeste studies will further validate QR-421a’s ability to stabilize vision loss in people with USH2A mediated retinitis pigmentosa and Usher syndrome.
The US Orphan Drug Designation would provide seven-year market exclusivity if Visomitin was approved for use in LHON.

Researchers at the University of Virginia School of Medicine have made a discovery linking lupus, a potentially debilitating autoimmune disorder, and macular degeneration, a leading cause of blindness.

According to the organization, the goal is to highlight and address the diverse and critical vision and eye health needs of children and to improve outcomes through advocacy, public health, education, and awareness.

Jay Duker, MD, discusses EYP-1901, EyePoint Pharmaceuticals’ sustained-release anti-VEGF drug for the treatment of wet AMD.

According to the company, OK-101 displays both anti-inflammatory and ocular pain-reducing potential.

At AAO 2021, Aerie Pharmaceuticals presented the results of their phase 2b clinical trial of AR-15512, a triple 8 antagonist, for the treatment of dry eye.

I-MED Pharma Inc., a Canadian-based firm, announced this week the expansion of its strategic partnership with ESW Vision in France to be their exclusive distributor in the United States.

TearLab Corp. announced that Adam Szaronos has been appointed president and CEO by the company's Board of Directors, effective immediately.
Novartis has revealed the first interpretable results from year two (week 100) of the Phase III KESTREL study.

Mohamed Abou Shousha, MD, PhD, discusses Heru’s wearable AI-powered diagnostic devices at AAO 2021.
Under terms of the agreement, ImprimisRx to assume full responsibility for US sales and marketing activities for Dexycu.

According to the company, the board will support the advancement of late-stage ophthalmic assets Nyxol and APX3330.

The company’s IOPerfect combines artificial intelligence in a VR-like headset to allow tele-diagnosis and remote monitoring of glaucoma.
The company will begin commercialization of the IC-8 small aperture IOL, used for cataract patients, upon successful completion of the manufacturing facility inspections and receipt of an official approval order from the FDA, which the company estimates in Q2 2022.

The agreement includes Europe, Commonwealth of Independent States countries, China, India, parts of Latin America and the Oceania countries.