Outlook Therapeutics has reported positive efficacy and safety data from Phase 3 NORSE TWO trial of bevacizumab-vikg.

Outlook Therapeutics has reported positive efficacy and safety data from Phase 3 NORSE TWO trial of bevacizumab-vikg.

The organization hopes to help 1 billion people worldwide, committing the international community to improve vision for 1.1 billion people living with preventable vision loss by 2030.
Investigators uncovered evidence that CaMKII could prove to be a desirable therapeutic target for vision preservation in conditions that damage the axons and somas of retinal ganglion cells.
A team of investigators used corneal confocal microscopy to identify corneal nerve damage in cases of long COVID.

This announcement was made at the 2021 ASCRS annual meeting.
AAO, Surgical Care Coalition teaming up to ensure the final version of the rule is fair to ophthalmologists and surgeons as one company is expressing its disappointment in the proposal.

Nanoscope Therapeutics Inc. has dosed its first patient in a late-stage Phase 2b trial of a gene therapy that delivers multi-characteristic opsin to retinal cells.
The Gilbert Family Foundation is collaborating with the Children’s Oncology Group and Children’s Hospital of Philadelphia to validate a tool to measure progressive vision loss.
According to iSTAR Medical, the STAR-V study will investigate the device in 350 patients with primary open angle glaucoma.

Alkeus Pharmaceuticals, Inc, announced Thursday that the FDA has granted Breakthrough Therapy Designation (BTS) for ALK-001 (C20-D3-vitamin A) for the treatment of Stargardt disease.
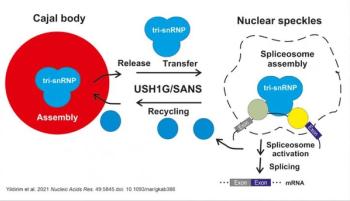
Usher syndrome 1G protein SANS regulates the splicing of genes, particularly those linked to Usher syndrome, which can lead to early vision loss form RP.

According to Unity Biotechnology, its trial for the small molecule inhibitor of Bcl-xL found improvement in visual acuity and central subfield thickness observed in diabetic macular edema and wet age-related macular degeneration patients treated with UBX1325.
The company, formerly called BELKIN Laser, announced Thursday that it will begin operating under the new name BELKIN Vision.
ASCRS has issued a statement calling on Aetna to drop a new prior authorization policy it implemented last week that delays cataract surgeries.

The American Medical Association recently announced policies adopted by physician and medical student leaders from all corners of medicine on the final day of the Special Meeting of the AMA House of Delegates.

The company’s IASAB will identify key business initiatives, ensuring they align with its strategic vision.
Santen Inc. announced Thursday that the U.S. FDA has approved Verkazia (cyclosporine ophthalmic emulsion) 0.1% eye drops to treat vernal keratoconjunctivitis (VKC).

If approved, PDS ranibizumab would become the first and only eye implant with continuous drug delivery offering people with wet AMD.

According to investigators, the system has been shown to almost triple the number of people with eye problems attending primary care, as well as increasing appropriate uptake of hospital services.

Oyster Point Pharma announced today the enrollment of the first participant in its OLYMPIA phase 2 clinical trial of OC-01 (varenicline) nasal spray to treat stage 1 neurotrophic keratopathy (NK).
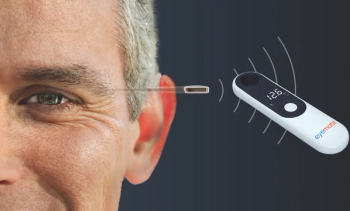
Implandata received FDA Breakthrough Device Designation for its Eyemate-SC in April and now has CE Mark approval in the European Union.

In recognition of Cataract Awareness Month, Fasika Woreta, MD, MPH, discusses IOL options for cataract patients.

According to investigators, the new technology is designed to detect telltale signs of major blinding diseases in retinal blood and tissue that typically go unseen until it is too late.

The US Supreme Court ruled Thursday that the Affordable Care Act remains valid, rejecting a claim by several states that a recent change to the law made it unconstitutional.
The American Academy of Ophthalmology is calling on its members to continue to build confidence in COVID-19 vaccines and encourage people to get vaccinated, including their staff.
Clearside Biomedical, Inc. reported today positive safety results from Cohort 1 of its ongoing OASIS phase1/2a clinical trial of CLS-AX for the treatment of wet AMD.
Classification criteria funded by the National Institutes of Health will facilitate clinical research for new therapies.

EyeGate Pharmaceuticals, Inc.'s lead product candidate is designed to treat dry eye disease-induced ocular surface inflammation.

Stuart will enroll 150 volunteers in the study, which is being conducted with the support of Stuart's strategic partner and CRO, Ora Clinical. ST-100 is based on PolyCol, the company's synthesized polypeptide tissue reparative platform.

Physicians want to make sure they see each their patient frequently enough not to miss important changes in eye disease.