
Researchers have now found that subjects who underwent cataract surgery had nearly 30% lower risk of developing dementia from any cause compared with those who did not.

Researchers have now found that subjects who underwent cataract surgery had nearly 30% lower risk of developing dementia from any cause compared with those who did not.

Verana Health showcases the VeraQ quality measures dashboard for the IRIS registry report at AAO 2021.

Study shows statistically significant improvement for primary endpoint of bulbar conjunctival hyperemia for OTX-DED 0.2 mg and 0.3 mg formulations compared with vehicle hydrogel insert.
The approval will allow LensGen to move forward with the IDE pivotal trial with the ultimate goal of seeking premarket approval in the United States.

AMA President Gerald E. Harmon, MD, said in a statement that President Joe Biden’s pandemic plan will make communities safer amid an evolving pandemic and confirmed cases of the Omicron variant.

Omidria will become a key product in Rayner's ophthalmology franchise, which includes intraocular lenses, ophthalmic viscoelastic devices and dry eye treatments.
The novel sustained-release technology has potential to eliminate the need for eye drops after cataract surgery.

Using its proprietary software, the company has been able to create models in a fraction of the time typically needed for fitting one.

The company's AI-enabled mobile telemedicine platform helps clinicians capture retinal images and screen for vision-threatening eye diseases, such as diabetic retinopathy, glaucoma and age-related macular degeneration.

The VIVID study focuses on three novel topical ophthalmic formulations under investigation for the treatment of presbyopia

The company’s re:Vive technology features 6 vision diagnostic exams, supported by 5 reimbursable CPT codes in 1 wearable solution.
Oxymetazoline hydrochloride has been recognized by the publication as a winner of its Best of Beauty Breakthrough Award.

The agreement also includes an exclusive option for Novartis to acquire Kedalion and its AcuStream technology. No financial terms were disclosed.
The Children’s Vision Equity Alliance was created to improve acces in children’s vision and eye health, working to advance equity in children’s vision and eye health through education, access, policies, and partnerships.

The 12-Month OMNI data from the prospective, multicenter GEMINI clinical trial show statistically significant postoperative reductions in mean IOP and suppression of daily IOP fluctuations.

Investigators from the University of Maryland found that uncovering the mechanism of vision loss in Usher syndrome yields additional drug targets for eventual development of better therapies.
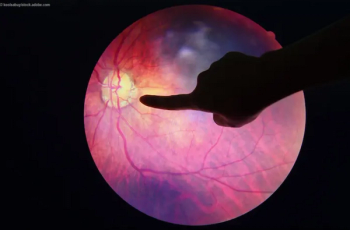
Unity Biotechnology announces improvement in visual acuity sustained through 24 weeks following single dose of UBX1325 in Phase 1 study of patients with advanced vascular eye disease.

Alcon this week announced its plans to acquire glaucoma surgery device maker Ivantis Inc. in a $475 million deal.

Organizations are urging consumers to be aware of insurance policies that can limit their access to sight-saving procedures and treatments.
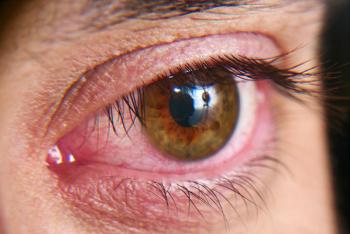
Aldeyra Therapeutics announced today that it has achieved the primary endpoint of ocular redness in its randomly assigned, double-masked, vehicle-controlled Phase 2 clinical trial in dry eye disease.

Allergan developed VUITY, which it noted is the first and only FDA-approved eye drop to treat presbyopia.

The Cleveland Clinic named Rishi Singh, MD, president of the Cleveland Clinic Martin North and South hospitals in Martin County, Florida.

Two photographs are selected by an independent panel of five judges for this year’s honors.
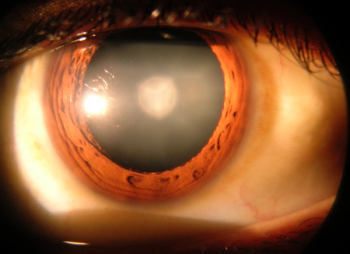
According to investigators, the risk of death from strokes, heart attacks and other vascular causes is increased by more than a third.

Bausch + Lomb and Clearside Biomedical announced Monday that the U.S. Food and Drug Administration has approved XIPERE (triamcinolone acetonide injectable suspension) for suprachoroidal use for the treatment of macular edema associated with uveitis.

According to the company, Susvimo is a first-of-its-kind therapeutic approach for wet AMD and may help people with the disease maintain their vision with as few as two treatments per year.

Fellowship recipient Christopher J. Kaler will explore functional links between BAP1 loss and aberrant activity of the nucleosome remodeling and deacetylase complex, which normally mediates embryonic lineage commitment and cellular differentiation.
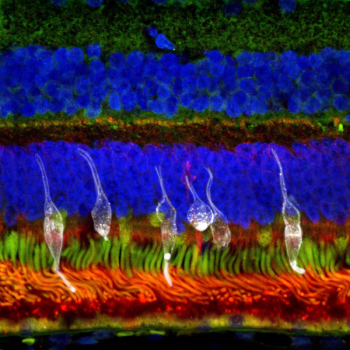
In a study, investigators demonstrated the safety and therapeutic potential of clinically compliant iPSC-derived photoreceptor precursors as a cell replacement source for future clinical trials.

Oyster Point Pharma, Inc. announced Monday that the U.S. FDA has granted approval of its TYRVAYA (varenicline solution) nasal spray 0.03 mg for the treatment of dry eye disease (DED), making it the first — and only — nasal spray approved for DED treatment in the U.S

Longtime clinician, researcher and head of the Ophthalmology Department at UCSF will succeed David W. Parke II, MD, at Academy helm.