
According to the company, it is developing elamipretide for treatment of extra-foveal GA under U.S. FDA Fast Track designation.

According to the company, it is developing elamipretide for treatment of extra-foveal GA under U.S. FDA Fast Track designation.

Dr. Katherine Talcott discusses baseline factors—CST thickness, hemoglobin A1C, and baseline vision—and their effects on DME resolution.

Dr. Carel Hoyng divulges the main takeaways from his Angiogenesis presentation, including the origins of Stargardt disease, correct diagnosis, ongoing gene therapy trials and the future of therapy.

Dr. Arshad M. Khanani reviews the Phase 2 part A results of the KALAHARI study of THR-149 for the treatment of DME.

During her talk at Angiogenesis, Dr. Loewenstein outlines how artificial intelligence could revolutionize diabetic retinopathy screening.
At Angiogenesis, Dr. SriniVas R. Sadda discusses how choriocapillaris may predict the rate of progression of atrophy.
Dr. Nadia K. Waheed reviews the latest updates on the FOCUS trial, evaluating AAV-based viral vector GT005 for the treatment of geographic atrophy.
Stanford researchers found an implanted chip, natural eyesight coordinate vision in study of macular degeneration patients.
A University of Montréal Hospital Research Center team reveals the fine mechanisms behind the major vascular defects observed in glaucoma patients and identifies new therapeutic targets.

According to the company, its C.STIM IPL system is based on intense pulsed light technology for the treatment of dry eye disease.

The Toronto-based medical eyewear company partnered with the visual assistance community to help empower an Oregon college student with enhanced vision.
Moore suffered from dry eye disease and Lumenis noted that treatment with OptiLight improved her condition. Now she’s partnering with the company to share her story and empower others.

The company completed enrollment ahead of schedule in a second Phase 3 FDA registration trial for Nyxol in RM with top-line results expected by the end of the first quarter.

The latest invention from Mark S. Humayun, MD, PhD, brings hope to sufferers of age-related macular degeneration, a common type of blindness.

NGM621 is a monoclonal antibody product candidate engineered to potently inhibit complement C3 for the treatment of patients with geographic atrophy secondary to age-related macular degeneration.
The company noted that the study was the first to evaluate whether AI software can accurately detect more-than-mild diabetic retinopathy using a single image per eye, obtained from either a desktop or handheld retinal camera.

The 3-month study will assess the safety and ocular hypotensive efficacy of TC-002 ophthalmic solution.

The FDA has approved the first generic of cyclosporine ophthalmic emulsion (Restasis; Allergan) 0.05% single-use vials of eye drops to increase tear production in patients with dry eye disease.

Cleveland Eye Bank Foundation (CEBF) will feature nine researchers from three eye institutions on February 15, from 3 to 5 pm Eastern, for its second annual virtual vision research symposium.

The company is expecting topline data in April from Saturn-2, the second Phase 3 pivotal trial of TP-03, with NDA submission on track for this year.

According to the companies, a social media initiative will include donation to Prevent Blindness’ Sight-Saving Fund.
The GMOPC was established in February 2020 at an inaugural meeting in Los Angeles, California, as an investigator-initiated clinical research study, with Heidelberg Engineering as an industry partner.
The university recently launched its Center for Neuronal Longevity, which brings together a multidisciplinary team to address unmet needs in vision loss and other neurological diseases.

Spect’s mobile device will enable providers to conduct critical eye screenings anywhere in a matter of minutes.

Faricimab is the first and only FDA-approved medicine targeting two distinct pathways, Ang-2 and VEGF-A, that often cause retinal diseases that may cause vision loss.

With the addition of 7 new therapeutics, Théa will expand its presence in the U.S. market.

A team of investigators at the University of California, Irvine have found that inhibiting ceramide accumulation in retina protects photoreceptors improves vision
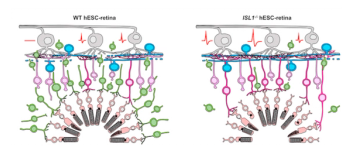
A team of investigators at the RIKEN Center for Biosystems Dynamics Research in Japan have used a genetic modification to improve human-derived retina transplants grown in the lab.

The historic eye institute celebrates six decades of leadership in eye care research, education and clinical practice.

Organization makes a commitment to expand translational research acceleration program and fund career development award.