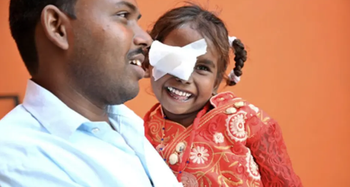
Research also highlights the top factors that lead to care, which can be used to improve parents' uptake of sight-saving surgery for their children.

Research also highlights the top factors that lead to care, which can be used to improve parents' uptake of sight-saving surgery for their children.

Work by a team of investigators is shedding light on the severity for gene variants and establishing outcome measures for therapeutic trials.
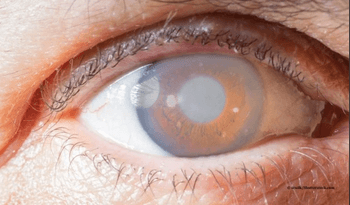
According to the company, the protocol enhancement for the RETeval Device marks an important step in mitigating vision loss from glaucoma.

Renewed consumer interest in and demand for LASIK, SMILE, and other procedures are among the key drivers for 2021 performance.
Nanoscope Therapeutics has received IND clearance from the FDA to begin a Phase 2 trial of its Multi-Characteristic Opsin ambient-light activatable optogenetic monotherapy to restore vision in Stargardt patients.

Across four studies, about half of eligible faricimab patients were able to go 4 months between treatments, and approximately three-quarters could be treated every 3 months or longer. Two papers published in The Lancet highlight one-year results.

A partnership that includes BALANCED Media|Technology, the Retina Foundation of the Southwest and Southern Methodist University is seeking a patent for machine learning software for OCT images aids in identity progression and treatment options

A team of investigators are working on a simple test that may someday identify those who can stop therapy.

As the omicron variant continues to surge across the country, putting staff at many practices on edge, waning immunity may be driving an increase in virus infections.
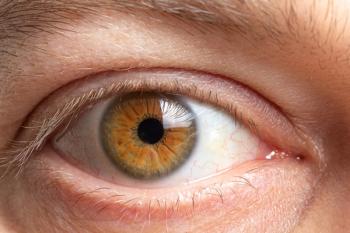
The difference between retina’s biological age and person’s real age linked to heightened death risk, with a team of Australian investigators finding that evidence suggests the microvasculature in the retina may be a reliable indicator of the overall health of the body’s circulatory system and the brain.

According to the companies, the agreement will open a channel between federal health facilities and CorneaGen's laboratories.

Since joining the foundation in 2018, Menzo has been involved in the formation of the Retinal Degeneration Fund, the venture arm of Foundation Fighting Blindness.

A University of California Davis study shows that small serving of the fruit increased protective pigments in the eye.

According to the company, biped.ai includes a comfortable and lightweight collar fitted with 3d cameras that continuously monitor a 170° field of view for the user detecting, tracking and predicting the trajectories of all surrounding elements a few seconds in advance.

According to researchers, gaining a good understanding of what Musashi proteins do and how to manipulate their function could lead to the development of a universal therapy for blinding diseases.
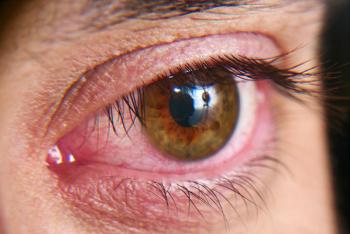
According to the company, A197 is a novel, first-in-class, topical immunomodulatory agent which will move into Phase II clinical trials in dry eye patients.

The pivotal trial of MINIject, STAR-V, now initiated in 13 sites across the US, has received positive feedback from glaucoma surgeons involved in the trial.

ASCENT, REGENXBIO’s Phase III clinical trial conducted in partnership with AbbVie, is expected to enroll patients in the United States and Canada, with pivotal trials expected to support BLA submission for RGX-314 in 2024.

AGTC-501 is a recombinant AAV vector-based gene therapy developed for the treatment of X-linked retinitis pigmentosa.

Marking Glaucoma Awareness Month this month, Bausch + Lomb and the Glaucoma Research Foundation have released key findings from a survey of glaucoma patients that was designed to gain a greater understanding of the impact that hyperemia can have in the treatment and lives of people living with glaucoma.

The acquisition brings the Hydrus Microstent into Alcon’s global surgical portfolio. The company will leverage its global footprint and leadership in ophthalmology to drive future growth, bringing the device to more patients worldwide.

According to the company, the iScan80 is a high-speed OCT system ideal for practices seeking an affordable and versatile OCT system.
According to the company, 4D-150 is a dual-transgene, intravitreal gene therapy designed to inhibit four distinct angiogenic factors to prevent angiogenesis and reduce vascular permeability for the treatment of wet AMD.

The gift will target expansion of the clinical space at Shiley Eye Institute, which opened 30 years ago with foundational support from the couple. It is part of a $27 million renovation and improvement project being undertaken by UC San Diego.
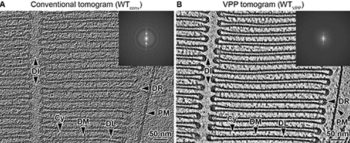
Team at University of California, Irvine provides an understanding of the mechanisms underlying the pathologies of certain gene mutations.

Ted Leng, MD, discusses his IRIS Registry analysis presentation regarding variations in vitreoretinal physician utilization of ancillary testing.
The novel gene therapy product candidate is being developed as a potential single intravitreal administration for blue cone monochromacy by delivering a functional copy of the OPN1LW gene.
Awards will be presented at the 11th Annual Focus on Eye Health National Summit, to be held virtually on July 13-14.

Electro-optical tech producer Gentex brings experience in automotive industry to healthcare, streamlining and enhancing production for eSight’s assistive medical devices.

The grant will provide Steven DeVries, MD, PhD, the opportunity to travel to Kyoto, Japan and learn from scientists at Ritsumeikan University about the process of growing 3D retinal organoids from stem cells, a technique first developed in Japan.