Dr. Carl Regillo discusses the 100-week results of KESTREL and KITE, two pivotal Phase 3 studies for brolucizumab for the treatment of diabetic macular edema.

Dr. Carl Regillo discusses the 100-week results of KESTREL and KITE, two pivotal Phase 3 studies for brolucizumab for the treatment of diabetic macular edema.

Dr. Arshad M. Khanani reviews the Phase 2 part A results of the KALAHARI study of THR-149 for the treatment of DME.

Most patients (95%) with the PDS implanted did not need supplemental treatment before the refills, indicating the persistence and durability of the treatment.

During her talk at Angiogenesis, Dr. Loewenstein outlines how artificial intelligence could revolutionize diabetic retinopathy screening.
After 2 years, the improvements in vision and anatomy were sustained with extended dosing out to every 16 weeks in a high percentage of patients.
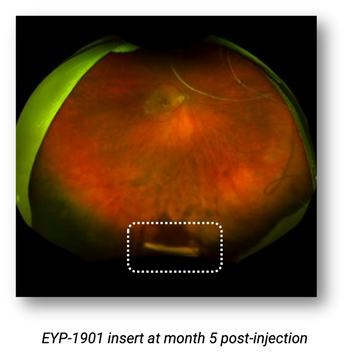
Jay S. Duker, MD, who presented data at the Bascom Palmer Eye Institute’s 19th annual Angiogenesis, Exudation, and Degeneration 2022 Virtual Edition, noted that previously treated patients showed a significantly reduced treatment
At Angiogenesis, Dr. SriniVas R. Sadda discusses how choriocapillaris may predict the rate of progression of atrophy.

During a presentation at the Bascom Palmer Eye Institute's Angiogenesis, Exudation, and Degeneration 2022 Virtual Edition conference, David S. Boyer, MD, explained that the drug, which is being considered to treat diabetic retinopathy, renal disease, and age-related macular degeneration, is intended to be an alternative therapy to monthly chronically administered intravitreal injections of anti-VEGF drugs.
Dr. Nadia K. Waheed reviews the latest updates on the FOCUS trial, evaluating AAV-based viral vector GT005 for the treatment of geographic atrophy.

In a presentation at the Bascom Palmer Eye Institute’s 19th annual Angiogenesis, Exudation, and Degeneration 2022 Virtual Edition, Amir Kashani, MD, PhD, discussed results that show the implant procedure is feasible on an outpatient basis and has a good safety and efficacy profile.
Dr. David S. Boyer describes how blocking Connexin-43 may improve the retinal vascular system function in patients with diabetes, potentially creating a future of oral medication for treatment of diabetic retinopathy and AMD.
Stanford researchers found an implanted chip, natural eyesight coordinate vision in study of macular degeneration patients.

The company completed enrollment ahead of schedule in a second Phase 3 FDA registration trial for Nyxol in RM with top-line results expected by the end of the first quarter.

The latest invention from Mark S. Humayun, MD, PhD, brings hope to sufferers of age-related macular degeneration, a common type of blindness.
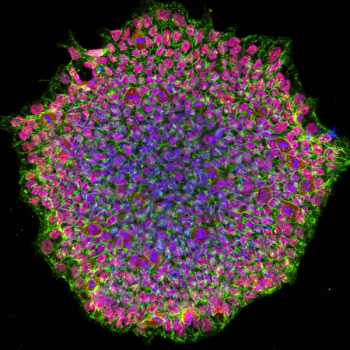
The team hopes the use of patient-derived stem cells will enable high-throughput drug screening for potential therapeutics for patients.

NGM621 is a monoclonal antibody product candidate engineered to potently inhibit complement C3 for the treatment of patients with geographic atrophy secondary to age-related macular degeneration.
The company noted that the study was the first to evaluate whether AI software can accurately detect more-than-mild diabetic retinopathy using a single image per eye, obtained from either a desktop or handheld retinal camera.
According to investigators, the implant can provide a sustained infusion of drug to the retina

A team of investigators has conducted ocular pharmacokinetic studies, including metabolism, providing valuable information for ocular drug development.

Cleveland Eye Bank Foundation (CEBF) will feature nine researchers from three eye institutions on February 15, from 3 to 5 pm Eastern, for its second annual virtual vision research symposium.

According to the companies, a social media initiative will include donation to Prevent Blindness’ Sight-Saving Fund.
The university recently launched its Center for Neuronal Longevity, which brings together a multidisciplinary team to address unmet needs in vision loss and other neurological diseases.

Spect’s mobile device will enable providers to conduct critical eye screenings anywhere in a matter of minutes.

Firas Rahhal, MD, discusses Outlook Therapeutics’ Phase 3 pivotal NORSE TWO trial for ONS-5010.

Faricimab is the first and only FDA-approved medicine targeting two distinct pathways, Ang-2 and VEGF-A, that often cause retinal diseases that may cause vision loss.