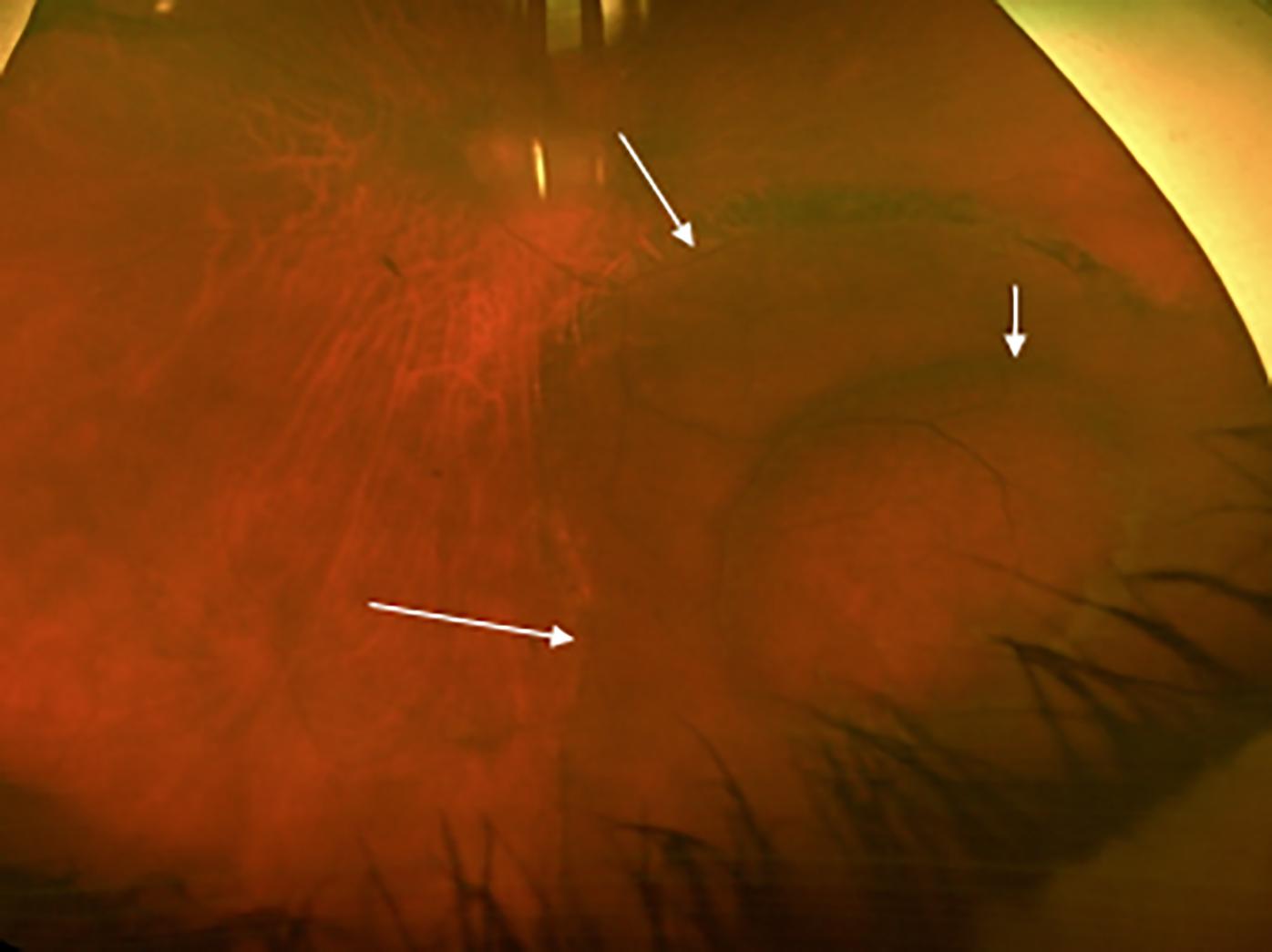
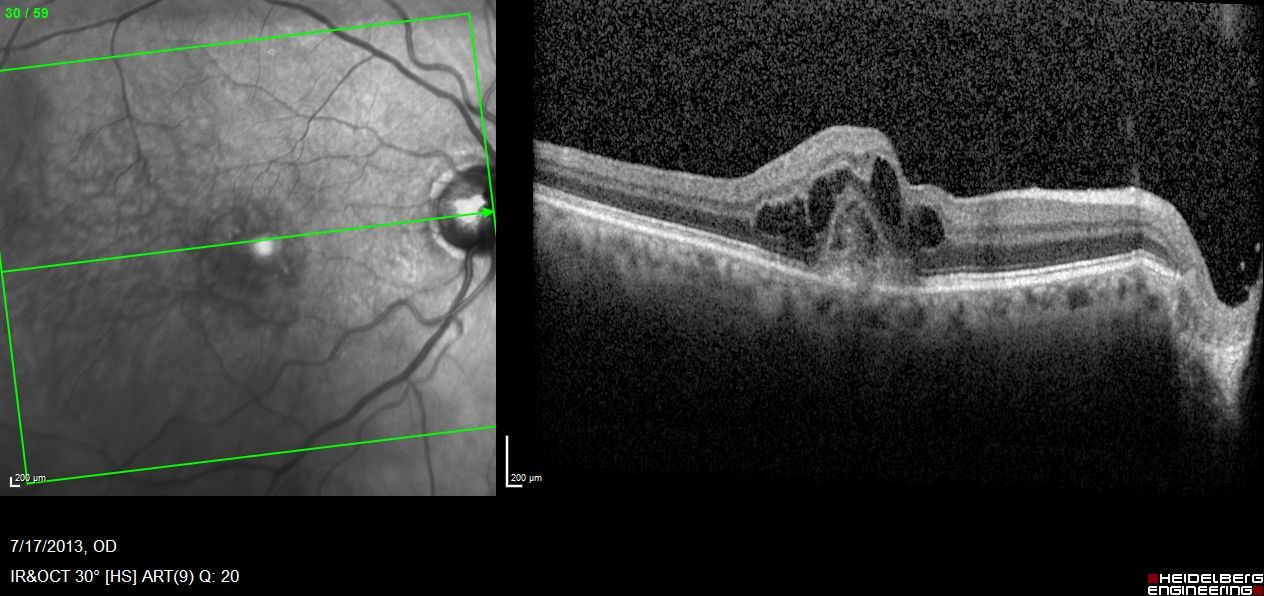
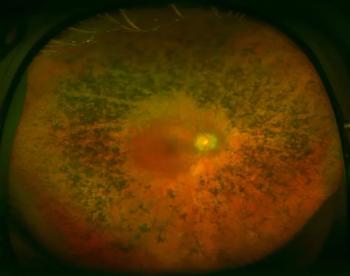
In a presentation at the American Society of Retina Specialists 2022 annual meeting at the Jacob K. Javits Convention Center in New York, Yoshihiro Yonekawa, MD, detailed that primary scleral buckling achieved the best visual acuity, and pointed out that rhegmatogenous detachments increased with age.