
"Tell Me Your Secrets" is the theme of the virtual April 2 event, which is expected to build on the foundation established during the first program held last fall.


"Tell Me Your Secrets" is the theme of the virtual April 2 event, which is expected to build on the foundation established during the first program held last fall.

Researchers from the Department of Ophthalmology at the University Hospital Bonn and Deutsches Zentrum für Neurodegenerative Erkrankungen suggest that assessments of the eye’s retina could help to detect a loss of brain substance, i. e. “brain atrophy.” The findings are based on data from the Rhineland Study.
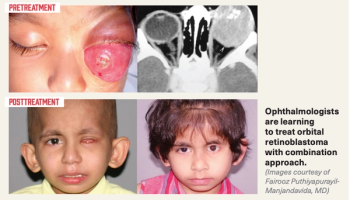
Investigators observe survival rate with orbital retinoblastoma improves substantially due to a combination approach that includes intensive sequential treatment comprised of chemotherapy, enucleation, and external beam radiation therapy.

According to the company, pegcetacoplan demonstrated continuous and clinically meaningful effects at month 18 in the studies, which also found that treatment effects in DERBY were comparable to OAKS during months 6 to 18. The combined 18-month data show the potential for improving treatment effects over.

Experts discuss the challenges regarding the treatment of retinal disorders, and how co-management can potentially address those challenges.
Dilsher S. Dhoot, MD, and A. Paul Chous, MA, OD, FAAO, discuss the co-management of retinal eye disorders and provide a clinical overview of the disease.
Mary Durbin, PhD, Chief Scientific Officer at Heru, discusses the benefits and capabilities of the various testing modalities available in 1 wearable platform.
Lisa M. Nijm, MD, JD, previews the program for this April 2 virtual event designed to help young ophthalmologists learn everything they need to know when starting practice.

Ultrasonic retinal prosthesis has been achieved by a research group at UCLA. It is a step towards a non-invasive retinal prosthesis that works without invasive eye surgery.

Investigators studying old flies have gained some new insight into retinal degeneration, seeking an understanding "of the molecular mechanisms that drive age-associated changes and the external and internal factors that influence them.”

Trial recruitment is enabled by the Foundation Fighting Blindness' patient registry, My Retina Tracker Registry, which includes gene

The clinical trial will evaluate the safety and tolerability of ADX-2191 in patients diagnosed with RP due to mutations of the rhodopsin gene, including the P23H gene mutation.
Rich Small, CEO of Neurotech, provides updates on the company’s pipeline that is the culmination of nearly 2 decades of research.

Christina Y. Weng, MD, MBA, an associate professor of ophthalmology and surgical retina fellowship program director at Baylor College of Medicine in Houston, recently shared some standout therapies for macular degeneration.

Aleksandra Rachitskaya, MD, discusses how the treatment landscape for inherited retinal diseases has changed and her hope for the future.

Dr. Quan Dong Nguyen reviews a Monte Carlo simulation that showed evidence that treating severe NPDR with anti-VEGF therapy garners positive results.

KSI-301, a therapy for patients with nAMD, did not meet the primary endpoint of showing non-inferior visual acuity gains compared to aflibercept given every eight weeks, however it was safe and well tolerated with no new or unexpected safety signals.


While there is currently no cure for blindness, an artificial vision system has undergone its first successful implantation, bringing with it the potential to restore partial vision to people who have lost their sight.

Tiffani Martin was diagnosed with type 1 juvenile diabetes when she was just 5 years old, and has battled myriad health issues as a result, including vision loss. Leading the charge for a multifactorial approach to combat diabetes, Kristen Nwanyanwu, MD, MBA, MHS, and her team have embraced the need for a multi-pronged program to address health disparities in diabetic retinopathy.
At Angiogenesis, Dr. David Brown presented the Phase 2 results of the CANDELA study for high dose aflibercept 8 milligram for wet AMD; here, he discusses those results.
The company recently took the wraps off an exclusive agreement with the Medical University of South Carolina (MUSC) Foundation for Research Development to license next generation complement-targeted gene therapies for the treatment of geographic atrophy and other ocular diseases.

Kato Pharmaceuticals will study the efficacy of Resolv ER for the treatment of vitreomacular attachment.
According to data presented by Horizon Therapeutics at the North American Neuro-Ophthalmology Society annual meeting held this week in Austin, Texas, Uplizna treatment is linked to fewer severe attacks and reduced levels of key disease-related biomarkers versus placebo.

Researchers know that parts of the retina are considered as biomarkers for Alzheimer’s, but the team from Otago’s Dunedin Multidisciplinary Health and Development Research Unit in New Zealand have been investigating the retina’s potential to indicate cognitive change earlier in life.