
Researchers in Trinity’s School of Genetics and Microbiology developed a new gene therapy, ophNdi1, that is the first of its kind to directly target mitochondrial function in cells that are malfunctioning in AMD.


Researchers in Trinity’s School of Genetics and Microbiology developed a new gene therapy, ophNdi1, that is the first of its kind to directly target mitochondrial function in cells that are malfunctioning in AMD.

According to the company, the clinical trial is reviewing EYP-1901, an investigational sustained delivery anti-vascular endothelial growth factor (anti-VEGF) treatment for wet age-related macular degeneration (wet AMD).
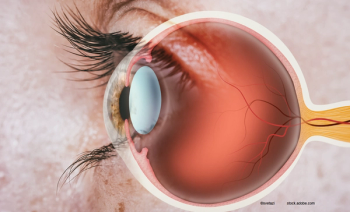
Retina indications for which ranibizumab-eqrn is interchangeable include neovascular (wet) age-related macular degeneration; macular edema following retinal vein occlusion; diabetic macular edema; diabetic retinopathy, and myopic choroidal neovascularization.
The doctors will join the Cataract and Primary Eye Care, Glaucoma, and Retina Services.

Positive results from recent pre-clinical studies support the potential of long-acting PASylated nomacopan to advance toward IND/IMPD for clinical trials in geographic atrophy (GA) in dry age-related macular degeneration (dAMD), a disease with no approved treatments.

According to the company, the study assesses the safety and tolerability of a single dose of CLS-AX administered to patients diagnosed with wet AMD by suprachoroidal injection.

LBS-008 is the company’s orally administered tablet for the treatment of Stargardt disease. There are currently no FDA approved treatments for Stargardt disease or dry AMD.
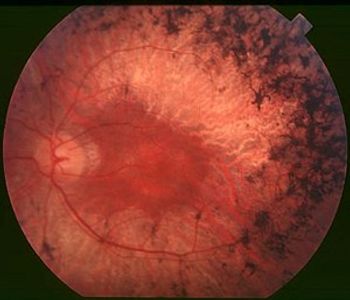
A team of scientists, led by Andrzej Foik, PhD, of the International Center for Translational Eye Research in Poland, is working on new therapies that may slow vision loss in patients diagnosed with retinal degeneration.

Eyes of mice lacking protective protein show signs similar to age-related macular degeneration.

The ASRS See for a Lifetime See a Retina Specialist initiative will spotlight early detection and outline the importance of seeing a retina specialist.

According to the company, pegcetacoplan is an investigational, targeted C3 therapy for the treatment of geographic atrophy secondary to age-related macular degeneration.
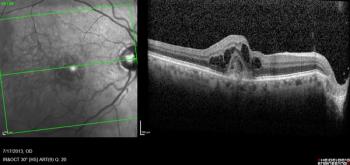
Speaking at the American Society of Retina Specialists 2022 annual meeting in New York, Carl Danzig, MD, detailed how treatment with faricimab in patients with neovascular AMD resulted in improvements in visual acuity, central subfield thickness and pigment epithelial detachments.

In the TENAYA and LUCERNE studies, more than 60% of faricimab patients could be treated every 4 months at 2 years, an increase from 45% at year 1. Study results are being presented at the American Society of Retina Specialists 2022 annual meeting in New York.

Mount Sinai study is among the first to link cardiovascular risk to a specific form of age-related macular degeneration.

The University of Oklahoma Health Sciences Center has received an unrestricted grant from Research to Prevent Blindness for $575,000 over 5 years to support eye research conducted by the Department of Ophthalmology.

Charles Brodowski, MD, and Patrick Rapuano, MD, have been named co-chief residents at Wills Eye Hospital, and will lead the team of resident doctors on staff, focusing on training and recruitment while attending to their own medical resident duties.

According to investigators, patients with age-related macular degeneration like the port delivery system for ranibizumab because it requires fewer treatments and there is less discomfort.
A team of investigators form Johns Hopkins Medicine say they’ve discovered that levels of a specific protein appears to help accurately predict whether people with the wet form of age-related macular degeneration may need lifelong, frequent eye injections to preserve vision or if they can be safely weaned off the treatments.

Investigators show the effectiveness of Home-OCT based remote monitoring model.
Investigators have found that recordings from the retina could identify distinct signals for both attention deficit hyperactivity disorder and autism spectrum disorder, providing a potential biomarker for each condition.


LumiThera Inc. noted that the trial results demonstrated statistically significant improvement in the prespecified primary endpoint in BCVA at 13 months in the PBM treatment group over the sham-treatment group.

Jason Menzo is named CEO, while Russell Kelley, PhD, MBA, will become managing director of the RD Fund. Ben Yerxa, PhD, will take the helm of Opus Genetics, the first RD Fund- and foundation-backed spin-off company.

Foundation Fighting Blindness is among a group of investors offering financial support for Nacuity’s efforts to stop oxidative tissue damage, a driver of blinding eye diseases.

Study demonstrates results of pegcetacoplan as a geographic atrophy therapy.