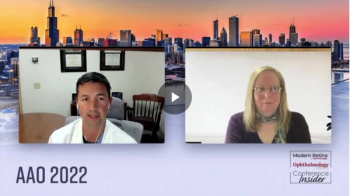
Nicole Bajic, MD, and Neel S. Vaidya, MD, MPH, share their insights from when they were at this crossroads. Learn more clinical pearls on topics like this and more at Real World Ophthalmology’s virtual conference, “Top 10 Things I Wish I Knew Sooner,” this Saturday, November 5.