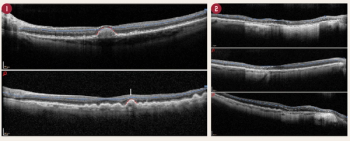
Alterations of the outer retinal layers from age-related macular degeneration (AMD) can interfere with the automated segmentation of the individual retinal layer thicknesses when using macular optical coherence tomography (OCT).

Alterations of the outer retinal layers from age-related macular degeneration (AMD) can interfere with the automated segmentation of the individual retinal layer thicknesses when using macular optical coherence tomography (OCT).

Jeff Cleland, PhD, CEO of Ashvattha, discusses safety data for an at-home subcutaneous injection option being developed for wet AMD and DME. The anti-VEGF candidate will enter a Phase 2 study later this year.

A discovery by investigators at the National Eye Institute sheds light on tissue targeted by age-related macular degeneration and other diseases.
The Eye Van, operated by Vision Loss Rehabilitation Canada, is a mobile medical clinic that delivers eye care in northern Ontario communities where ophthalmology services aren't readily available.

As more and more practices embrace dark adaptation testing, AdaptDx technology has become a staple in primary eye care.

The OpRegen trial is a cell therapy trial, looking to explore potential safety and efficacy for patients with advanced dry age-related macular degeneration (AMD).

Variable TULP1 missense mutations in inherited retinal diseases.
The intravitreal implant (OTX-TKI) is being evaluated to treat wet age-related macular degeneration in a phase 1b clinical trial.

According to the ALOFT study, patients demonstrate improved long-term vision in real-world setting after wet AMD conversion compared to current standard of care.

If the BLA is approved, the company could receive 12 years of marketing exclusivity for an FDA-approved alternative for the most frequently used anti-VEGF treatment in wet AMD patients in the United States.

The company is planning a Phase 2 trial with an optimized formulation in wet AMD that is expected to start in fourth quarter of 2022.

According to the company, pegcetacoplan demonstrated continuous and clinically meaningful effects at month 18 in the studies, which also found that treatment effects in DERBY were comparable to OAKS during months 6 to 18. The combined 18-month data show the potential for improving treatment effects over.
Mary Durbin, PhD, Chief Scientific Officer at Heru, discusses the benefits and capabilities of the various testing modalities available in 1 wearable platform.

Ultrasonic retinal prosthesis has been achieved by a research group at UCLA. It is a step towards a non-invasive retinal prosthesis that works without invasive eye surgery.

Investigators studying old flies have gained some new insight into retinal degeneration, seeking an understanding "of the molecular mechanisms that drive age-associated changes and the external and internal factors that influence them.”

Trial recruitment is enabled by the Foundation Fighting Blindness' patient registry, My Retina Tracker Registry, which includes gene
Rich Small, CEO of Neurotech, provides updates on the company’s pipeline that is the culmination of nearly 2 decades of research.

Christina Y. Weng, MD, MBA, an associate professor of ophthalmology and surgical retina fellowship program director at Baylor College of Medicine in Houston, recently shared some standout therapies for macular degeneration.

Aleksandra Rachitskaya, MD, discusses how the treatment landscape for inherited retinal diseases has changed and her hope for the future.
At Angiogenesis, Dr. David Brown presented the Phase 2 results of the CANDELA study for high dose aflibercept 8 milligram for wet AMD; here, he discusses those results.

AM712 is a novel bispecific biologic molecule specifically designed for ocular use. In pre-clinical studies, it demonstrated efficacy, ocular pharmacokinetics, and the desired safety profile supporting clinical exploration.

During a presentation at the Bascom Palmer Eye Institute Angiogenesis, Exudation, and Degeneration 2022 conference, Robert Bhisitkul, MD, PhD, provided 24-week clinical data from the Phase 1 study, demonstrating that patients with DME and wAMD showed improved visual acuity through 24 weeks following a single dose of UBX1325.
Dr. Carl Regillo discusses the 100-week results of KESTREL and KITE, two pivotal Phase 3 studies for brolucizumab for the treatment of diabetic macular edema.

Most patients (95%) with the PDS implanted did not need supplemental treatment before the refills, indicating the persistence and durability of the treatment.
At Angiogenesis, Dr. SriniVas R. Sadda discusses how choriocapillaris may predict the rate of progression of atrophy.