
AMD
Latest News
Latest Videos

CME Content
More News
Japanese investigators may have found a way of predicting which patients will develop intraocular inflammation in retrospective study.

The expert panel discusses what constitutes treatment failure in anti-VEGF therapy for nAMD and examines how various treatments may yield varying results in managing PED.

Adrienne Scott, MD, presents a case of neovascular AMD with PED in a patient who experiences a stroke while on anti-VEGF therapy.

The company announced key secondary endpoints were achieved with both EYP-1901 doses. These include a more than 80% reduction in treatment burden, with nearly two-thirds of eyes supplement-free up to 6 months.

The ranibizumab biosimilar is a recombinant antigen-binding fragment (Fab).

Expert retina specialists deliberate on their approach to treating DME, emphasizing their primary treatment choice and cases where they would use PRP laser therapy and steroids alongside anti-VEGF treatments.

Carl Danzig, MD, presents a case involving a patient with both DME and PDR, showcasing the impressive anatomic improvement achieved by treating with a dual anti-VEGF and Ang2 inhibitor.
By joining the group of physicians, researchers, and industry partners working together to define optimal biomarkers and endpoints in AMD, RetinAI hopes to forge a global retinal imaging initiative targeting research in the disease.
Rishi Singh, MD, discusses the criteria for identifying patients who would benefit from biosimilar therapy.

Rishi Singh, MD, presents a case of neovascular age-related macular degeneration and diabetic macular edema demonstrating how the use of ranibizumab biosimilars can yield remarkable anatomical improvements comparable to those expected with reference biologics.

Rishi Singh, MD, provides an overview of biosimilars in the context of retinal diseases and discusses their significance within the healthcare system.
Mohammed Genead, MD, CEO of Aviceda spoke with the Ophthalmology Times team about the company's Phase II/III SIGLEC trial part 1 results, which were shared at this year's American Academy of Ophthalmology meeting.

Previously, the EMA had approved Eylea in a dosage of 2 mg. After considering the EMA’s recommendation, the European Commission will decide whether to issue final approval of the drug.

ABP 938 is an investigational biosimilar to EYLEA® (aflibercept).
The purpose of this investigation was 2-fold: to estimate the costs of treating GA with pegcetacoplan and to identify possible utility measures to compare treatments for GA.

The clinical trial will evaluate the safety and efficacy of CLS-AX (axitinib injectable suspension), a tyrosine kinase inhibitor. According to the company, topline data is expected during the third quarter of 2024.
According to the company, a change from base line best corrected visual acuity based on an average at week 44, 48 and 52 serves as the primary endpoint.
According to a news release, the J-code for SYFOVRE will become effective on October 1, 2023.

According to the Louisiana State University Health Sciences Center, elovanoids have been shown to restore the structure and integrity of damaged photoreceptor cells by repairing, remodeling and regenerating healthy cells.
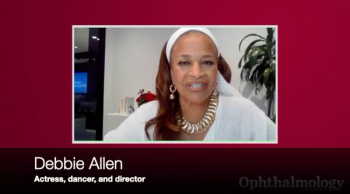
Debbie Allen, an award-winning actress, dancer, choreographer, singer-songwriter, director and producer is teaming up with Prevent Blindness and Regeneron for the Gr8 Eye Movement, an awareness campaign that aims to educate and encourage those who are at risk of certain retinal diseases to prioritize their eye health.

"What research at the 2023 ASRS meeting do you find exciting or interesting?" Here's what Aaron Lee, MD, Megan Baldwin PhD, and Carl Danzig, MD had to say.

We asked, "What research at ASRS 2023 do you find exciting or interesting?" Here's what Paul Hahn, MD, PhD, Kerrie Brady, BPharm, MBA, MS, and Michael Singer, MD had to say!

According to Regeneron, the approval was based on data in the PULSAR and PHOTON trials, in which the drug demonstrated clinically equivalent vision gains to aflibercept Injection 2 mg that were maintained with fewer injections.
According to the company, the MAA submission is based on data from the GATHER1 and GATHER2 Phase III clinical trials.

The study met its primary efficacy endpoint for the neovascular age-related macular degeneration (nAMD) treatment.






























