Behin Barahimi, MD, discusses some current trends in the treatment of dry eye disease. An oculoplastic, orbital and reconstructive surgeon, she is a member of the faculty at Vanderbilt Eye Institute.

Behin Barahimi, MD, discusses some current trends in the treatment of dry eye disease. An oculoplastic, orbital and reconstructive surgeon, she is a member of the faculty at Vanderbilt Eye Institute.
Oyster Point Pharma Inc. this week announced the expansion of patient access to varenicline solution (Tyrvaya) nasal spray and provided an update on the commercial performance of the spray in the United States. According to the company, its expanded patient access programs include more eligible patients with dry eye disease.
Scientists at the Louisiana State University Health New Orleans Neuroscience Center of Excellence have developed a new, experimental human cell line from retinal pigment epithelial cells.

Treatment and reduces burden of care, providing a treatment option for patients diagnosed with diabetic macular edema.

MyMD Pharmaceuticals Inc. announced recently that it has entered into a material transfer agreement with Bascom Palmer Eye Institute of Miami, Florida to collaborate on a pre-clinical study using MYMD-1 as a potential treatment for traumatic optic neuropathy.

According to the company, the study assesses the safety and tolerability of a single dose of CLS-AX administered to patients diagnosed with wet AMD by suprachoroidal injection.
Results from the U.S. pivotal trial showed Apthera IOL subjects achieved statistically superior uncorrected intermediate and near vision, and equivalent distance vision and contrast sensitivity compared to control subjects.

According to the company, 6-month safety and efficacy data are expected in Q1 2023. MCO-010 gene therapy reprograms healthy retinal cells to make them photosensitive.
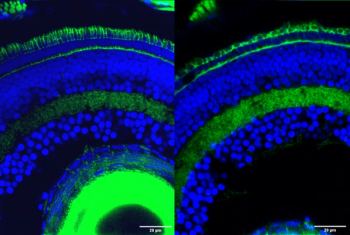
The findings could lead to a new understanding of unexplained causes of heritable retinal diseases.

LBS-008 is the company’s orally administered tablet for the treatment of Stargardt disease. There are currently no FDA approved treatments for Stargardt disease or dry AMD.
NIH-funded clinical trial finds that starting with a cheaper drug and switching to a more expensive drug as needed leads to good vision outcomes in diabetic macular edema.
According to a team of investigators from Yale, Stanford and Dartmouth, the variances in regional healthcare spending are not limited to either public or private payers.
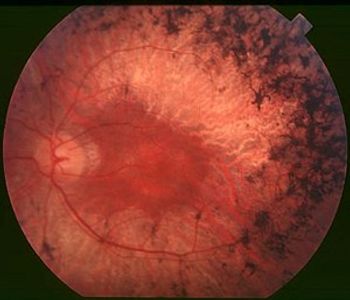
A team of scientists, led by Andrzej Foik, PhD, of the International Center for Translational Eye Research in Poland, is working on new therapies that may slow vision loss in patients diagnosed with retinal degeneration.

The companies will jointly develop 4D bio-fabricated corneal transplants for diseases that require endothelial keratoplasty and natural lenticule transplants.

Eyes of mice lacking protective protein show signs similar to age-related macular degeneration.

The ASRS See for a Lifetime See a Retina Specialist initiative will spotlight early detection and outline the importance of seeing a retina specialist.

According to the company, pegcetacoplan is an investigational, targeted C3 therapy for the treatment of geographic atrophy secondary to age-related macular degeneration.

Charles Wykoff, MD, took the stage at the American Society of Retina Specialists annual meeting in New York to present a talk titled “Suprachoroidal Delivery of RGX-314 Gene Therapy for Diabetic Retinopathy: Phase II ALTITUDE Study.” He discusses results of the trial, which showed improvements for patients diagnosed with diabetic macular edema and non-proliferative diabetic retinopathy, with notable improvements according to the Diabetic Retinopathy Severity Scale.

A national poll suggests most parents overlook simple steps to protect children’s eyes; 1 in 7 parents say their child has not had a vision test in 2 years.

The company and its U.S. joint venture partner and licensee, HLB Therapeutics, signed a letter of intent this week with a global ophthalmology contract research organization to conduct a pair of phase 3 clinical trials simultaneously beginning in the fall in the U.S. and Europe for patients with neurotrophic keratitis.

University of Chicago Medical Center investigators reviewed 2 years of data on assaults with paintball guns found more patients than expected suffered vision-threatening emergencies after being struck in the eye, with several experiencing a rupture of the eyeball or even permanent blindness.
Children may find it difficult to return to play or school after a concussion, and ophthalmologists and pediatricians can help identify if some will need special learning accommodations or additional referral for treatment.

According to the company, the study found a positive safety and tolerability profile for aCT1 for treatment of corneal injury.

Researchers at the University of Strathclyde have developed an affordable device which takes 3D images and could shift the landscape of eye screening and treatment around the world.

Researchers have found that social media use among physicians jumped during the COVID-19 pandemic, but the role it plays in networking, mentorship and support among ophthalmologists remains unknown.

In the TENAYA and LUCERNE studies, more than 60% of faricimab patients could be treated every 4 months at 2 years, an increase from 45% at year 1. Study results are being presented at the American Society of Retina Specialists 2022 annual meeting in New York.

The first in a series of upgrades to Stellaris Elite incudes maximum vacuum increase and trocar/cannula system removeable valve cap enhancement.

Kala Pharmaceuticals Inc. announced that it has completed the sale of its commercial portfolio and related intellectual property assets to Alcon Inc., a transaction that was first announced in May.
Lloyd Clark, MD, highlights his ASRS presentation focusing on the PANORAMA study detailing the use of aflibercept in the management of diabetic retinopathy.