Dry Eye
Latest News


Successful phase 2 trial, Aerie Pharmaceuticals, Inc.’s dry eye candidate advancing to phase 3
Latest Videos

CME Content
More News
Top-line results are expected during second quarter for the therapeutic to treat patients with dry eye disease.
Cynthia Matossian, MD, shares her thoughts on the premium patient "experience" or "journey," emphasizing the outcomes of patient experiences.

According to the company, VVN001 demonstrated clinical and statistical superiority over vehicle in reducing total and sub-regional corneal fluorescein staining scores.

Raj Kannan, the new CEO of Aerie Pharmaceuticals, talks with Ophthalmology Times' David Hutton about what's coming down the pipeline for Aerie.
Short-term steroid use can alleviate patient discomfort.

While investigators have found a link between dry eye and depression, the exact reasons behind the link are not immediately known.

The company expects to have topline proof of concept data by the end of the first quarter of the year.

TearSolutions Inc. is preparing to conduct two trials, one in a primary Sjögren’s Syndrome patient population and the second in a potentially less severe general dry eye population for the treatment of DED.

Cynthia Matossian, MD, FACS, ABES, points out that there has been confusing news lately about what is (and is not) covered by Medicare when it comes to in-office treatments for meibomian gland dysfunction.

According to the company, its C.STIM IPL system is based on intense pulsed light technology for the treatment of dry eye disease.
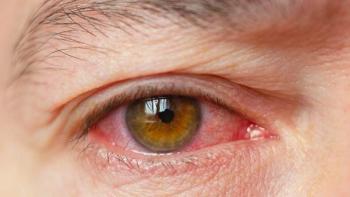
Moore suffered from dry eye disease and Lumenis noted that treatment with OptiLight improved her condition. Now she’s partnering with the company to share her story and empower others.

The FDA has approved the first generic of cyclosporine ophthalmic emulsion (Restasis; Allergan) 0.05% single-use vials of eye drops to increase tear production in patients with dry eye disease.

A new treatment option may increase basal tear production in patients.
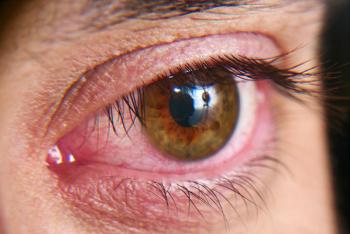
According to the company, A197 is a novel, first-in-class, topical immunomodulatory agent which will move into Phase II clinical trials in dry eye patients.

According to the company, OK-101 displays both anti-inflammatory and ocular pain-reducing potential.

According to Lisa Nijm, MD, JD, patients can benefit from short-term, on-label treatment with a novel loteprednol formulation

At AAO 2021, Aerie Pharmaceuticals presented the results of their phase 2b clinical trial of AR-15512, a triple 8 antagonist, for the treatment of dry eye.

I-MED Pharma Inc., a Canadian-based firm, announced this week the expansion of its strategic partnership with ESW Vision in France to be their exclusive distributor in the United States.

Investigators are hopeful that future research can focus on public health concerns of dry eye disease.

Study shows statistically significant improvement for primary endpoint of bulbar conjunctival hyperemia for OTX-DED 0.2 mg and 0.3 mg formulations compared with vehicle hydrogel insert.
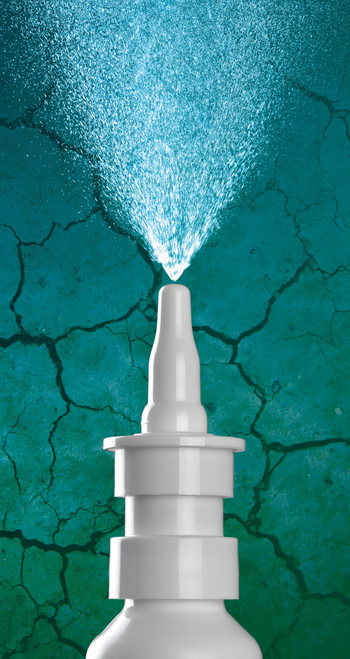
Product stimulates basal tear production, restores tear film homeostasis, and relieves symptoms.

Aldeyra Therapeutics announced today that it has achieved the primary endpoint of ocular redness in its randomly assigned, double-masked, vehicle-controlled Phase 2 clinical trial in dry eye disease.

Oyster Point Pharma, Inc. announced Monday that the U.S. FDA has granted approval of its TYRVAYA (varenicline solution) nasal spray 0.03 mg for the treatment of dry eye disease (DED), making it the first — and only — nasal spray approved for DED treatment in the U.S

Investigators find that estradiol drops can ease signs and symptoms of DED.

A team of investigators at the Texas Tech University Health Sciences Center have developed a technology derived from corneal epithelial stem cells to improve outcomes for DED patients.