
Stephen C. Pflugfelder, MD, honored as the recipient of the Binkhorst Lecture and medal at ASCRS 2019, lectured on the quest for tear stability in dry eye patients.

Stephen C. Pflugfelder, MD, honored as the recipient of the Binkhorst Lecture and medal at ASCRS 2019, lectured on the quest for tear stability in dry eye patients.
Many medical specialties share medications and technologies, from various classes of drugs to diagnostic testing and surgical instruments. Here are just a few “adopted” technologies we’re happy to see in the hands of ophthalmologists.
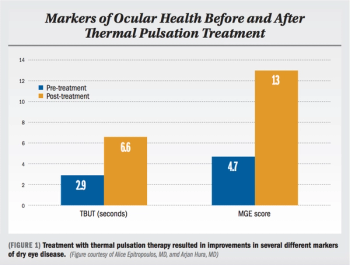
Thermal pulsation therapy has potential to improve gland structure in most patients.
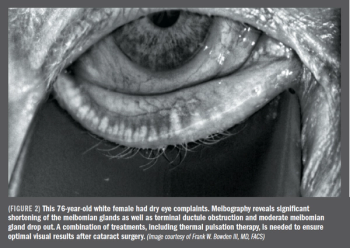
Start ocular surface treatment 6 weeks prior to cataract surgery for best outcomes
Study and use of platelet preparation, other biologics viewed as next frontier

BRM421 safe, well tolerated in controlled adverse environment model
First treat pre-existing conditions, such as medication toxicity, eyelid disease
Phase II proof-of-concept study indicates efficacy signals at day 28 and day 84
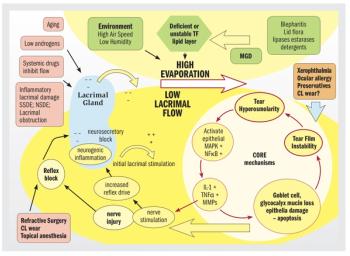
New diagnostic modalities have enhanced clinical abilities, whereas some standbys still get the job done
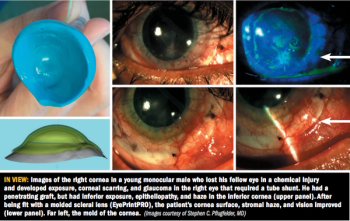
Management evolves from nasal neurostimulation, prosthetic replacement of ocular surface ecosystem
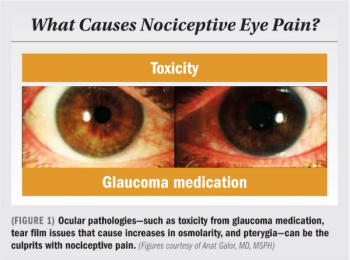
Look beyond ocular surface dysfunction for clues, causes of dry eye pain
Some doctors worry that once they make the initial dry eye diagnosis, they’ll be faced with the tough reality of treating it effectively. Luckily, positive results are possible with certain therapies.

The holiday season brings lots of good cheer and also worsens dry eyes-here's how your patients can effectively manage their symptoms.

Defining role of topography, tear film osmolarity, other tests

IPL + thermal pulsation: A thorough approach to dry eye

For weary patients, dry eye clinics are often the last stop. Here, they finally find the knowledge, understanding, diagnosis, and treatment they deserve. Intense pulsed light (IPL) therapy, an important and practice-differentiating component of treatment, has helped us provide long-term relief from signs and symptoms.

Letters to the Editor may be submitted to [email protected]. Letters may be edited for clarity and length.
Laura M. Periman, MD, shares why dry eye spurs her curiosity, and why being a “dry eye sleuth” is refreshing (yes, refreshing!) in today’s medical practice environment.



Christopher J. Rapuano, MD, explains how red eye might be something more. Superior limbic keratoconjunctivitis may be identified by lifting the upper lid and having the patient look down.
As the diversity of drop products expands and knowledge of the nuances of various ocular surface maladies increases, consider how the differences between drop products might make one drop a better choice for a given patient.