Dry Eye
Latest News
Latest Videos

CME Content
More News

RAFARM and BioNanoSim have entered into an agreement to create the company.

According to the company, CyclASol is preservative-free and does not contain any oils or surfactants, which can be irritating and disturbing for the tear film.

The topical drug is being tested in a multi-center, double-masked, placebo-controlled trial to treat dry eye disease (DED).

According to the company, the Preservative Freedom Coalition will put the topic of preservative-free eye care in focus through education to eye care professionals and patients.

New option a potential treatment for patients diagnosed with dry eye disease.

In a recent update, the CDC has noted an additional death, bringing the death toll up to 4, with numerous counts of vision loss across the US.
The company noted tanfanercept did not demonstrate statistical significance in either of the primary outcome measures of improvement in central corneal staining score or in improvement in Eye Dryness Score assessed at week 8 in subjects with dry eye disease, compared to vehicle. However, tanfanercept did demonstrate a highly statistically significant improvement on one of the secondary outcome measures, Schirmer testing of tear volume.
Ophthalmology Times reached out to members of its editorial advisory board along with ophthalmologists who specialize in dry eye for their comments on what this approval means for their patients with dry eye.

According to the companies, MIEBO is the first and only prescription eye drop approved for dry eye disease that directly targets tear evaporation, based on consistent results from a pair of pivotal Phase 3 trials.

In November 2022, doctors in Cleveland diagnosed a patient with a corneal ulcer with a Pseudomona aeruginosa infection. The patient acquired the infection from tainted EzriCare eye drops months before the CDC’s February 2023 warning.

From ingredients that should not be included to ingredients that should be but are not, and improper storage and manufacturing, numerous eye drops have raised safety concerns.

Lucie Moore, BSc, in a presentation at the American Society of Cataract and Refractive Surgery annual meeting in San Diego, said the study focused on identifying factors that could put patients at a higher risk for developing dry eye disease.

According to the company, consistent clinical efficacy across multiple signs and symptoms of dry eye along with excellent safety and tolerability build a differentiating product profile.

Instead, vitamins C and E may actually be associated with a slightly increased risk of DED.

Esen Akpek, MD, sat down with David Hutton, Managing Editor, Ophthalmology Times®, to discuss ocular surface disease and the lifestyle choices that could affect eye health. As well as what The Tear Film & Ocular Surface Society is doing to promote research on the topic.

The ESSENCE-2 study showed a significantly greater reduction in the total corneal fluorescein staining (tCFS) score.

Alice Epitropoulos, MD, goes over some tips and methods to educate patients about OSD before performing any surgery.

Dirty equipment and sterilization issues are among the many violations found by the FDA.

The month will serve to educate the public on a variety of women’s vision issues.

Ehsan Sadri, MD, FACS, and William Trattler, MD, highlight some of the pending FDA approvals and PDUFA dates in the anterior segment sector and what these products will mean for ophthalmologists and their patients.

Long-term screen users and contact lens wearers in group face challenges.

According to the companies, NOV03 consistently met primary endpoints for the signs and symptoms of dry eye disease linked with Meibomian gland dysfunction. A PDUFA data is set for June 28.

Avenova Eye Health Support oral supplement aims to comfort dry eyes and support overall eye health.
The company’s Purely Soothing eye drop is used as an anti-inflammatory used to assist with symptoms of ocular irritation and/or swelling, including dry eye.
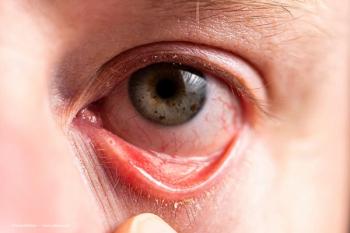
Alice Epitropoulos, MD, gives some insight into meibomian gland dysfunction (MGD), one of the most prevalent forms of dry eye.