Dry Eye
Latest News
Latest Videos

CME Content
More News

Reservoir restoration can re establish the normal anatomy and function of the conjunctiva.
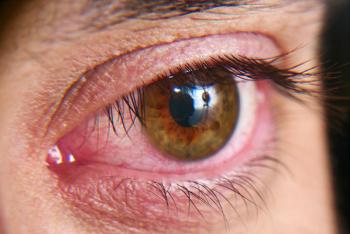
By creating customized packages that incorporate the range of diagnostic and treatment options available, practices can simplify dry eye management and improve patients' outcomes.

Oyster Point Pharma Inc. today announced that the largest Medicare pharmacy benefit manager in the United States will add its varenicline solution nasal spray (Tyrvaya) for the treatment of dry eye on its Medicare Part D formularies, effective September 1.
An independent data monitoring committee recommends continuing study with a sample size target of up to 350 Patients. According to the company, no safety concerns have been identified and topline results are expected during the second quarter of 2023.

ThermaMEDx today announced a partnership with EyeCare Partners to expand EverTears distribution across the network of the national provider of clinically integrated eye care.

Tarsus Pharmaceuticals has enrolled the first patient in a Phase 2a clinical trial studying TP-03 for the treatment of meibomian gland disease in patients diagnosed with Demodex mites.
Behin Barahimi, MD, discusses some current trends in the treatment of dry eye disease. An oculoplastic, orbital and reconstructive surgeon, she is a member of the faculty at Vanderbilt Eye Institute.

Oyster Point Pharma Inc. this week announced the expansion of patient access to varenicline solution (Tyrvaya) nasal spray and provided an update on the commercial performance of the spray in the United States. According to the company, its expanded patient access programs include more eligible patients with dry eye disease.

Cynthia Matossian, MD, FACS, ABES, explains why eye care professionals should oversee the selection and use of artificial tears for patients with dry eye.

According to the company, the study found a positive safety and tolerability profile for aCT1 for treatment of corneal injury.
Kala Pharmaceuticals Inc. announced that it has completed the sale of its commercial portfolio and related intellectual property assets to Alcon Inc., a transaction that was first announced in May.

NOV03 is an investigational treatment with a proposed indication of treating the signs and symptoms of dry eye disease associated with Meibomian gland dysfunction.

The organization hopes to increase awareness and education of a condition that affects vision, mental health.

RegeneRx Biopharmaceuticals Inc. said its U.S. joint venture partner and licensee, HLB Therapeutics, will expand its ophthalmic clinical development program with RGN-259 in two indications, neurotrophic keratopathy and dry eye disease.

The treatments typically include application of heat, lid hygiene, and massage of eyelid.
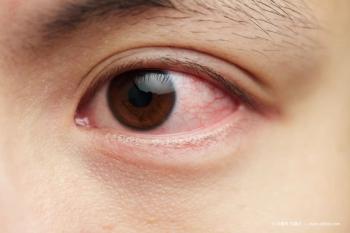
One obvious trend has been that dry eye disease is a concurrent problem for virtually all my patients, and we need to address it for optimum comfort and surgical results.

The Phase 3 registrational study is examining AR-15512, a differentiated, novel product candidate for the treatment of the signs and symptoms of Dry Eye Disease

According to the companies, Kala will receive $60 million in upfront payment, and could be eligible to receive additional sales-based milestone payments.
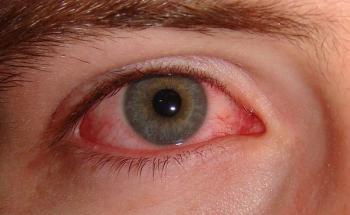
In a paper, investigators identified strategies used by patients to reduce the cost of therapy and its impact on adherence to treatment Patients may be reluctant to disclose challenges regarding adherence to dry eye disease therapy, as well as fears of worsening quality of life.

BRIM Biotechnology Inc. has entered into a new strategic partnership with Ora Inc. for the late-stage clinical development of lead drug candidate BRM421 for dry eye syndrome (DES).

The study's primary endpoints were to look at increasing Schirmer's scores, as well as eye discomfort score.

Takeaway: there doesn't seem to be a specific dry eye phenotype that does better or worse.
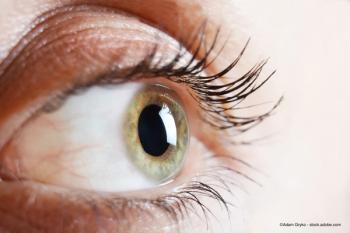
Ophthalmologists, optometrists gain nonpharmacological treatment options for some of the eye’s most complex issues, providing better outcomes.

Patients are at risk for developing severe dry eye and ocular surface disease.

Michelle Senchyna, PhD, VP Clinical Development and Medical Affairs for Aerie Pharmaceuticals, gives a company update on the various clinical trials in the pipeline.