Dry Eye
Latest News
Video Series

Latest Videos
CME Content
More News

An overview of the top 10 stories from Optometry Times in 2025.

Panel examines drug mechanisms, patient factors, and strategies to optimize results.

Aldeyra met with the FDA on December 12, 2025, in which the FDA requested the company submit the CSR from the reproxalap dry eye disease field trial to the NDA.

The company plans to submit its NDA package in the second half of 2026.

The grading scales were compiled with the goals of achieving conciseness and the ability to provide clear drug-dose-modification recommendations compared with the previous ocular CTCAE scale.

Of the 1000 patients with dry eye symptoms surveyed, 80% stated that “they would try a science-backed eye drop that mimics natural tears and restores tear-film balance."

Cyclosporine ophthalmic emulsion 0.05% is a topical immunomodulator to increase tear production in patients whose tear production is thought to be reduced due to ocular inflammation associated with dry eye syndrome.

HUC1-394 is a peptide-based eye drop for dry eyes being developed by the company.

Researchers analyze the DREAM study, revealing lid margin collarettes in dry eye patients and their surprising lack of progression over time.

Ophthalmologists weigh in on evolving diagnostic tools, emerging therapies, and practical approaches for managing today’s most challenging ocular-surface cases.

Host Deborah Ristvedt, DO, is joined by Marguerite B. McDonald, MD, FACS, to discuss her journey with pioneering contributions to ophthalmology, patient-centered care, leadership, and guidance for the next generation.

A study reveals diverse ocular symptoms in Sjögren’s disease, linking eye features to oral and pain manifestations for personalized treatment strategies.

Lupin notes that the acquisition will help to strengthen the company by “integrating VISUfarma’s established commercial operations.”

This marks the first time that the TearCare System has been included in the TFOS DEWS III Management and Therapy Report.
A novel use of minor salivary glands offers lasting relief and visual improvement.
New findings confirm TearCare's long-term effectiveness for dry eye disease, showing significant patient improvements with minimal treatments over 2 years.

TearCare is associated with greater health utility over time but also resulted in significant cost savings compared with CsA

The solution was approved by the US Food and Drug Administration in late May 2025.
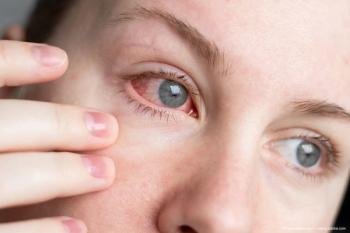
RTP-008 features sustained immunosuppressant delivery for long-term therapeutic effects.

A Prescription Drug User Fee Act (PDUFA) target action date of December 16, 2025, was assigned by the FDA.

The tool is designed to help with the application of mono-dose, dry eye eyedrop vials more easily and accurately.

MHRA issues precautionary recall for Zaditen eye drops due to potential microbial contamination risk
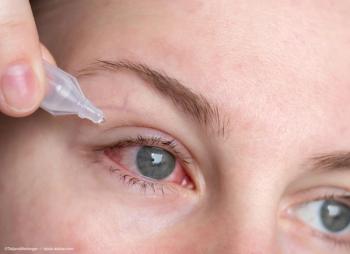
In addition to Prevent Blindness' usual free resources, the organization is providing a dedicated web page, fact sheets, and graphics in English and Spanish.