
According to the company, the AI platform can predict a person's risk of having a stroke or a heart attack in the next five years.

According to the company, the AI platform can predict a person's risk of having a stroke or a heart attack in the next five years.

According to Novartis, the approval is based on year 1 data from the Phase III KESTREL and KITE clinical trials investigating brolucizumab-dbll 6 mg vs aflibercept 2 mg in diabetic macular edema patients.

Faricimab is the first treatment for wet AMD and DME in Canada that acts by targeting both VEGF-A and Ang-2, two key drivers of vascular instability that have been associated with vision-threatening retinal conditions.

The company noted that pegcetacoplan, designed to regulate excessive activation of the complement cascade, part of the body’s immune system, which can lead to the onset and progression of many serious diseases, was granted Fast Track designation by the FDA for the treatment of geographic atrophy.

The study will investigate the safety, tolerability, pharmacokinetics, and efficacy of AM712 in subjects with neovascular age-related macular degeneration.

Outlook Therapeutics initially filed the BLA fin March or the use of bevacizumab-vikg in the treatment of wet AMD. The company said it wlll re-submit a revised BLA by September.

The Phase 3 registrational study is examining AR-15512, a differentiated, novel product candidate for the treatment of the signs and symptoms of Dry Eye Disease

According to the company, the ReCLAIM-2 study of elamipretide demonstrates a correlation between ellipsoid zone dysfunction and vision.

According to the company, a clinical trial evaluating Nyxol eye drops for night vision disturbances met FDA-agreed primary endpoint with subjects gaining 3 lines of low-contrast distance vision under dim light conditions compared to placebo.

According to the companies, Kala will receive $60 million in upfront payment, and could be eligible to receive additional sales-based milestone payments.
Two grants will be available, each offering $150,000 to scientists focusing on glaucoma research who have already received their first independent federal National Institutes of Health (NIH) grant and are collecting new and novel data to apply for a second R01.

Philip Ruzycki, PhD, receives a career development award that will provide $350,000 to support his laboratory over the next 4 years.

Formosa Pharmaceuticals, AimMax Therapeutics unveiled data from CPN-301, the first of two pivotal Phase 3 clinical studies of APP13007, a novel ophthalmic nanosuspension formulation of a potent corticosteroid, clobetasol propionate (0.05%).

The company is partnering with leading ophthalmology practices focused on improving patient outcomes. OMNY solutions are derived from electronic health record data and provide valuable information on the evolving treatment paradigm for patients with ophthalmic disease, including difficult to obtain insights on the rationale behind treatment decisions, and the impact of social determinants of health on care delivery and outcomes.

The American Academy of Ophthalmology applauded Congress for reaching the 290-consponsor milestone for the proposal. By reaching the two-thirds majority of bipartisan support in Congress, the bill is eligible for inclusion on the Consensus Calendar under new rules that were established in 2019.

As more and more practices embrace dark adaptation testing, AdaptDx technology has become a staple in primary eye care.

The company's VialConnect is an intuitive clinical trials management platform built for coordinators and investigators.

The companies will collaborate to commercialize therapeutic option in Greater China, South Korea and Select Southeast Asian Markets for the treatment of presbyopia, expanding access to long-acting, presbyopia-correcting eye drops for approximately 600 million patients.
The research, conducted by Boston Medical Center and Stanford University School of Medicine, found that most of the mistreatment came from patients and visitors.

The 10-week Flying Eye Hospital program will offer courses focusing on advanced glaucoma and simulation lectures for residents.
According to the company, the trial did not demonstrate efficacy on the key clinical endpoints and will not advance THR-687 to Part B of the trial. As a result, Oxurion will now shift its focus to the Phase 2 development program for THR-149, which recently demonstrated a compelling safety and efficacy profile for the treatment of patients with DME.

Aurion noted that its first candidate is a cell therapy for the treatment of corneal edema secondary to endothelial dysfunction.

Bath was recognized as the first Black woman physician to receive a medical patent, as well as the first woman to lead a post-graduate training program in ophthalmology. She joins four other inventors as the most recent inductees into the Hall.

In research sponsored by Skye Bioscience Inc., investigators at the University of Mississippi have demonstrated stronger and longer-lasting reduction of intraocular pressure when a proprietary molecule, SBI-100, is formulated as a nanoemulsion containing the mucoadhesive agent Carbopol 940.

TP-03 met the primary and all secondary endpoints, effectively resolved Demodex blepharitis and was well-tolerated with no serious treatment-related adverse events. 56% of patients on TP-03 achieved the primary endpoint of complete collarette cure.
Researchers at the University of Southern California have stimulated the retinas of blind mice using focused ultrasound technology.

This advancement reinforces Alcon’s commitment to surgeon training and education, as part of the Alcon Experience Academy. The new device will be introduced at the upcoming ASCRS meeting and surgeons will be able to experience the new technology firsthand.
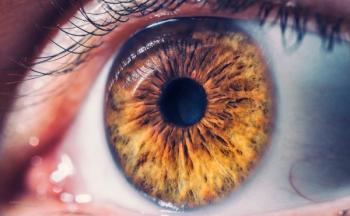
The award recipients were chosen by an all-volunteer selection committee. The committee consists of leaders and professionals in the ophthalmology, optometry, advocacy, public health and scientific communities.

Impact of native lipids on rhodopsin signaling and regeneration opens door to GPCR drug discovery in native membrane environments

The trial marks the first-ever in vivo delivery of an experimental CRISPR gene editing medicine to a pediatric patient, with the company on track to complete dosing of the pediatric mid-dose cohort in the first half of 2022.