CMS is proposing to cut the Medicare conversion factor—the basic starting point for calculating Medicare payments—by 4.42% for CY2023.

CMS is proposing to cut the Medicare conversion factor—the basic starting point for calculating Medicare payments—by 4.42% for CY2023.
A team of investigators form Johns Hopkins Medicine say they’ve discovered that levels of a specific protein appears to help accurately predict whether people with the wet form of age-related macular degeneration may need lifelong, frequent eye injections to preserve vision or if they can be safely weaned off the treatments.
NOV03 is an investigational treatment with a proposed indication of treating the signs and symptoms of dry eye disease associated with Meibomian gland dysfunction.
Researchers at the University of Bristol, as part of a 10-year follow-up study, found that severe “brain bleeds” experienced by some babies in the first year following their birth lead to long-term sight problems.

Kiora Pharmaceuticals announced it has enrolled the first patient as a part of a Phase 2 study evaluating KIO-201 topical eye drops in patients with PCED, a rare ocular surface condition characterized by non-healing wounds on the eye surface.

A team of investigators at the Agency for Science, Technology and Research in Singapore have developed a bio-functional thermogel, a type of synthetic polymer, to prevent retinal scarring caused by failed retinal detachment repair surgery.

A new deep learning model that can identify disease-related features from images of eyes has been unveiled by a group of investigators from Tohoku University.

The organization hopes to increase awareness and education of a condition that affects vision, mental health.

Investigators show the effectiveness of Home-OCT based remote monitoring model.
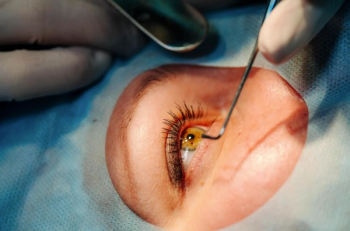
ASCRS, AAO behind year-long drive for policy change, with Aetna dropping its requirement on July 1 for pre-authorization for cataract surgery, covering patients in 48 of 50 states.

OCS-01 is being reviewed for the treatment of inflammation and pain after cataract surgery, with the potential to become the first once-daily, topical and preservative-free corticosteroid for treating inflammation and pain following ocular surgery.

The FDA has accepted the supplemental Biologics License Application of aflibercept injection for review in patients with diabetic retinopathy.

According to the company, the primary outcome of the MGT009 study is safety, and botaretigene sparoparvovec treatment was found to be generally safe and well-tolerated.
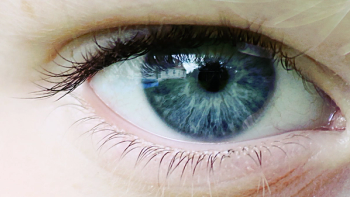
Investigators have found that recordings from the retina could identify distinct signals for both attention deficit hyperactivity disorder and autism spectrum disorder, providing a potential biomarker for each condition.

A team of investigators from the Yong Loo Lin School of Medicine at the National University of Singapore has found that there is a bidirectional association between chronic kidney disease and glaucoma.

RegeneRx Biopharmaceuticals Inc. said its U.S. joint venture partner and licensee, HLB Therapeutics, will expand its ophthalmic clinical development program with RGN-259 in two indications, neurotrophic keratopathy and dry eye disease.

A team of investigators at Flinders University in Australia have found that a specific cell within the retina appears to be particularly good at housing Ebola and other viruses.


LumiThera Inc. noted that the trial results demonstrated statistically significant improvement in the prespecified primary endpoint in BCVA at 13 months in the PBM treatment group over the sham-treatment group.

The U.S. Centers for Medicare and Medicaid Services code is effective July 1 and will enhance access to Xipere, the only therapy available in the United States for suprachoroidal use in the treatment of macular edema associated with uveitis.
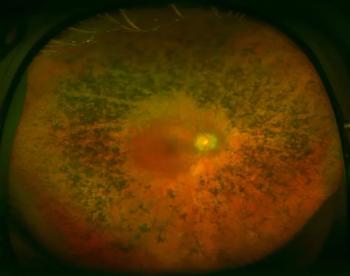
A team led by researchers from the University of Geneva has identified a molecular mechanism that causes degeneration of the eye’s photoreceptors, which can lead to blindness.

Jason Menzo is named CEO, while Russell Kelley, PhD, MBA, will become managing director of the RD Fund. Ben Yerxa, PhD, will take the helm of Opus Genetics, the first RD Fund- and foundation-backed spin-off company.

A study by Orbis International found that children with myopia experienced significantly higher levels of depression and anxiety than their peers without vision impairment.

Foundation Fighting Blindness is among a group of investors offering financial support for Nacuity’s efforts to stop oxidative tissue damage, a driver of blinding eye diseases.
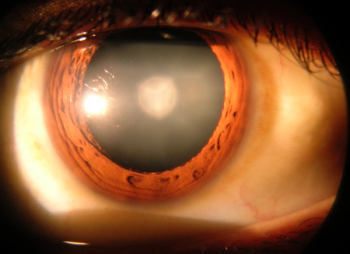
According to the organization, the eBook content is designed for residents, ophthalmologists, fellows, medical students and other professionals performing or supporting cataract surgery.

The treatments typically include application of heat, lid hygiene, and massage of eyelid.
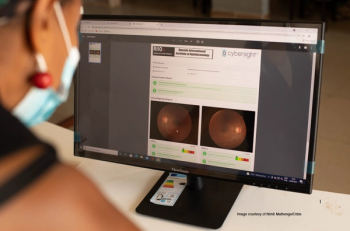
The research from eye care nonprofit Orbis International focuses on diabetic retinopathy in Rwanda, where associated vision complications from diabetes are growing.

The systems could be in the hands of surgeons in the third quarter through a targeted initial launch, according to the company.

According to investigators, a key reason for the link may lie in circadian “clocks,” the molecular machinery within every cell of every organism, which have evolved to adapt to daily stresses, such as changes in light and temperature caused by the rising and setting of the sun.

The company announced it has received FDA IND clearance for linsitinib and prepares to initiate Phase 2b clinical trial.