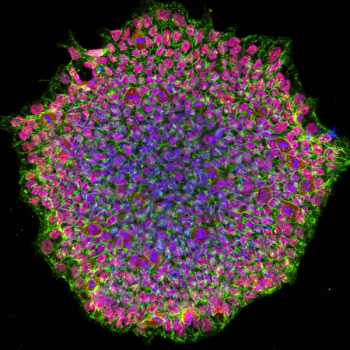
The team hopes the use of patient-derived stem cells will enable high-throughput drug screening for potential therapeutics for patients.

The team hopes the use of patient-derived stem cells will enable high-throughput drug screening for potential therapeutics for patients.

NGM621 is a monoclonal antibody product candidate engineered to potently inhibit complement C3 for the treatment of patients with geographic atrophy secondary to age-related macular degeneration.
The company noted that the study was the first to evaluate whether AI software can accurately detect more-than-mild diabetic retinopathy using a single image per eye, obtained from either a desktop or handheld retinal camera.

The 3-month study will assess the safety and ocular hypotensive efficacy of TC-002 ophthalmic solution.

The FDA has approved the first generic of cyclosporine ophthalmic emulsion (Restasis; Allergan) 0.05% single-use vials of eye drops to increase tear production in patients with dry eye disease.
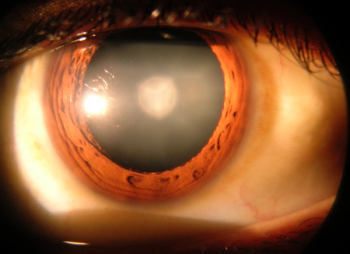
Researchers at the University of Arizona Health Sciences will use a $1.6 million National Eye Institute grant to study age-related lens cell changes that can lead to cataracts.
According to investigators, the implant can provide a sustained infusion of drug to the retina

Preeya Gupta, MD, walks through her four pearls for successful corneal collagen cross-linking, including handling patient expectations, managing the epithelial cells and preventing infectious keratitis.

A team of investigators has conducted ocular pharmacokinetic studies, including metabolism, providing valuable information for ocular drug development.

A team of investigators examined the association between COVID-19 transmissibility and elapsed time since infection, and found that SARS-CoV-2 may still be transmissible 10 days post-infection.

Cleveland Eye Bank Foundation (CEBF) will feature nine researchers from three eye institutions on February 15, from 3 to 5 pm Eastern, for its second annual virtual vision research symposium.

The future of this age-related condition is bright and in focus.

According to the companies, a social media initiative will include donation to Prevent Blindness’ Sight-Saving Fund.
The GMOPC was established in February 2020 at an inaugural meeting in Los Angeles, California, as an investigator-initiated clinical research study, with Heidelberg Engineering as an industry partner.
A new treatment option may increase basal tear production in patients.
The university recently launched its Center for Neuronal Longevity, which brings together a multidisciplinary team to address unmet needs in vision loss and other neurological diseases.

Spect’s mobile device will enable providers to conduct critical eye screenings anywhere in a matter of minutes.

New routes of therapeutic administration are paving the way for innovative applications.

Faricimab is the first and only FDA-approved medicine targeting two distinct pathways, Ang-2 and VEGF-A, that often cause retinal diseases that may cause vision loss.

Joshua Mali, MD, shares how the FDA-approved faricimab-svoa (Vabysmo, Genentech) will change the treatment landscape for wet AMD and DME.

The study reviews the outcomes following penetrating, closed trauma–related injuries.

The historic eye institute celebrates six decades of leadership in eye care research, education and clinical practice.

Organization makes a commitment to expand translational research acceleration program and fund career development award.
Research also highlights the top factors that lead to care, which can be used to improve parents' uptake of sight-saving surgery for their children.
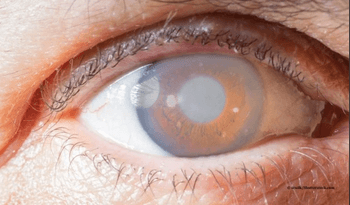
According to the company, the protocol enhancement for the RETeval Device marks an important step in mitigating vision loss from glaucoma.

Investigators find that successful outcomes may depend on the awareness of red-flag symptoms and signs.

Renewed consumer interest in and demand for LASIK, SMILE, and other procedures are among the key drivers for 2021 performance.

According to the companies, the trial will evaluate the safety, tolerability, and immune response of the vaccine that can be administered as a primary shot and a booster.

This Bascom Palmer Eye Institute 2-day virtual scientific program promises a distinguished international faculty who will share the latest developments in imaging, translational research, and clinical trials as well as the impact these advances will have on clinical ophthalmology.
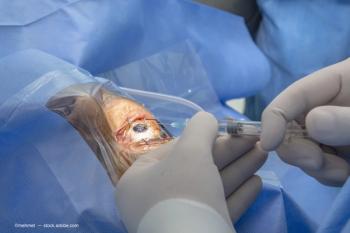
Nanoscope Therapeutics has received IND clearance from the FDA to begin a Phase 2 trial of its Multi-Characteristic Opsin ambient-light activatable optogenetic monotherapy to restore vision in Stargardt patients.