
Impact of native lipids on rhodopsin signaling and regeneration opens door to GPCR drug discovery in native membrane environments

Impact of native lipids on rhodopsin signaling and regeneration opens door to GPCR drug discovery in native membrane environments

The trial marks the first-ever in vivo delivery of an experimental CRISPR gene editing medicine to a pediatric patient, with the company on track to complete dosing of the pediatric mid-dose cohort in the first half of 2022.

The challenge has been in early detection of inflammation. Matrix metalloproteinase-9 (MMP-9), an inflammatory biomarker consistently elevated in the tears of dry eye patients, may accelerate early diagnosis when detected.
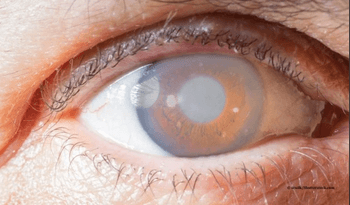
NCX 470 is currently enrolling patients in two multi-regional Phase 3 glaucoma clinical trials, Mont Blanc and Denali. The objective of these two trials is to demonstrate statistically superior IOP lowering of once daily dosed NCX 470
Severe ocular involvement demands inflammation control

Three prestigious lectures are featured, including Ehud Assia, MD, from Israel who gave the ESCRS Binkhorst Lecture; Richard Packard, MD, from England who gave the Choyce Lecture to the UKISCRS; and Michael Snyder, of the Cincinnati Eye Institute in Cincinnati, Ohio, USA, who delivered the Kelman Lecture at the 2021 American Academy of Ophthalmology meeting.

According to the company, the trial will study the effect of the drug in treatment-naïve patients with diabetic macular edema (DME) who are members of underrepresented patient populations.

A team of investigators from Pohang University of Science and Technology has found that conjunctival goblet cell examination is important for the precise diagnosis and effective treatment of ocular surface diseases; however, CGC examination has not been possible until now due to lack of non-invasive devices.

According to researchers at the University of California, Irvine, base editing may provide long-lasting retinal protection and prevent vision deterioration in patients with inherited retinal degeneration, specifically in Leber congenital amaurosis patients.

Investigators find technology aids instruction of medical students

The data support the ongoing development of KPI-012, a novel investigational secretome therapy, to address the complex wound healing process in persistent corneal epithelial defect (PCED).
Orbis International celebrates 40 years of innovation this year, beginning in 1982 with their iconic flying eye hospital. Since their start, Orbis has continued their innovation, working to achieve sustainable and scalable impact in the countries where they work. Doris Macharia, MD, senior vice president of global programs with Orbis, talks with Ophthalmology Times' Sheryl Stevenson, reflecting on the last 40 years and what's to come.
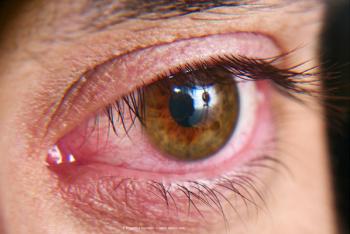
Top-line results are expected during second quarter for the therapeutic to treat patients with dry eye disease.
The company’s Systane iLux2 Meibomian Gland Dysfunction Thermal Pulsation System is a handheld device that allows patients to see the need for treatment and visualize their procedure.

According to the company, the VIRGO trial evaluated the safety and efficacy of the investigational twice-daily administration of pilocarpine HCl ophthalmic solution 1.25% in adults with presbyopia. The trial met its primary efficacy endpoint, the company noted.

According to the company, the modifier gene therapy candidate is for the treatment of retinitis pigmentosa resulting from mutations in the nuclear receptor subfamily 2 group E member 3 and Rhodopsin genes.
Device demonstrates potential as a durable, sustained-release glaucoma therapy

The International Agency for the Prevention of Blindness (IAPB) recently announced an expansion of their ‘Focus on Glaucoma’ and ‘Focus on Diabetes’ series with their new ‘Focus on Child Eye Health’ series in partnership with CooperVision.
The company announced the expansion of its commercial rollout of its MIGS implant to the UK

Investigators at the International Center for Materials Nanoarchitectonics have developed the first-ever artificial retinal device that increases the edge contrast between lighter and darker areas of an image, using ionic migration and interaction within solid.
Despite inherent issues, clinicians still perform the procedure
April is Presbyopia Awareness Month. Brieann K. Adair, OD, of NYU Langone Health, speaks with Ophthalmology Times’ Sheryl Stevenson about the importance of Presbyopia awareness, treatment options and more.

In those first few months, I realized there were so many aspects of real-life, post-residency practice we had never been taught during medical school or residency.
A University of Houston study found that minorities have fewer eye exams, higher instances of disease.

If the BLA is approved, the company could receive 12 years of marketing exclusivity for an FDA-approved alternative for the most frequently used anti-VEGF treatment in wet AMD patients in the United States.

A separate analysis will be presented at the American Academy of Neurology 2022 Annual Meeting, showing that UPLIZNA reduced pan associated with NMOSD over 3 years.
Bausch + Lomb and Clearside Biomedical Inc. are rolling out the new therapeutic, approved by the FDA for suprachoroidal use for the treatment of macular edema associated with uveitis, a form of eye inflammation.

Beginning in the 1970s, James J. Salz, MD, was part of a group of ophthalmologists who first inserted intraocular lens implants during cataract surgery.

The American College of Medical Genetics and Genomics Foundation for Genetic and Genomic Medicine grants its Next Generation Fellowship Awards to promising early-career professionals in a range of medical genetics and genomics specialties. The awards were presented at the 2022 ACMG Clinical Genetics Meeting.

The company is planning a Phase 2 trial with an optimized formulation in wet AMD that is expected to start in fourth quarter of 2022.