
Electro-optical tech producer Gentex brings experience in automotive industry to healthcare, streamlining and enhancing production for eSight’s assistive medical devices.

Electro-optical tech producer Gentex brings experience in automotive industry to healthcare, streamlining and enhancing production for eSight’s assistive medical devices.

The new procedure eliminates the need for scleral flaps by positioning the T-shaped IOL haptics in the scleral wall.

Clear communication, on-call services, and listening to your patients may keep them from expensive and often unnecessary emergency room visits.
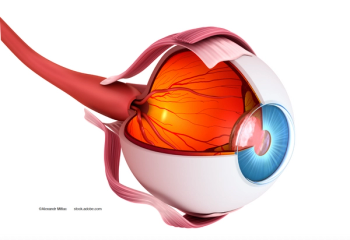
Consecutive intravitreal dexamethasone treatments may be beneficial for patients with DME patients who had undergone a previous vitrectomy.

Delayed presentations and acute shortage of donor corneal tissues for emergency keratoplasty are among reasons given for adverse outcome.
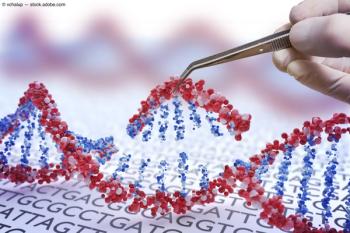
Researchers have found that study participants with healthy eyes and no history of AMD had thinner retinas if they carried the genes that put them at risk.

For 72 straight hours, the study volunteer lay in a bed at UT Southwestern, the monotony broken only at night when researchers placed his lower body in a sealed, vacuum-equipped sleeping bag to pull down body fluids that naturally flowed into his head while supine.
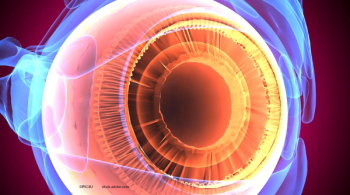
The improvement became evident during an average time of 6 months.

In light of another unprecedented year filled with technological advancements and pivots as a result of the pandemic, Joshua Mali, MD, offers his top 5 predictions in ophthalmology for 2022.

The approach seems to provide high levels of spectacle independence and patients' satisfaction, with limited complications associated.
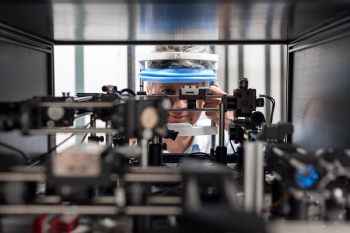
A team of scientists have developed a two-photon excited fluorescence scanning laser ophthalmoscope, an instrument that allows viewing the biochemistry of vision in the living eye in real time.
The Viterbi Family Department of Ophthalmology and Shiley Eye Institute, both part of UC San Diego Health, received funding from the Nixon Visions Foundation that will support studies of the PRPH2 gene linked to macular dystrophy and boost stem cell research aimed at developing early diagnosis and a cure.

Approach shows potential as a promising second-line screening tool for patients with diabetes.

With every 10-year increase in patient age, the risk of the patients needing concomitant administration of steroid eye drops decreased by half, according to investigators.

A team of researchers with the West Virginia School of Medicine are studying how a benign virus can make new treatments for eye diseases possible.

A team of researchers from Thomas Jefferson University have found that immune cells could be doing much more than first thought in protecting our eyes.

A virtual, risk-based approach to preoperative medical evaluations for cataract surgery may be associated with safe and efficient outcomes, according to study investigators.
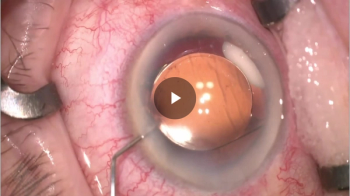
Robert H. Osher, MD, shows the merits of drug delivery via capsular bag after cataract surgery.

The cell-by-cell atlas will help in the study of eye disorders and development of cell therapy to replace damaged eye tissue.

According to the company, a suprachoroidal injection of CLS-AX 0.1 mg dose was well-tolerated in cohort 2 with no treatment related adverse events, while the consistent safety profile observed in in cohorts 1 and 2 supports advancement to cohort 3.

Analysis finds acceptable outcomes can be expected when pterygium surgery is performed by a supervised ophthalmology resident.

Researchers found there was, on average, a 17% improvement in participants’ color contrast vision when exposed to three minutes of deep red light in the morning.

Spectacle independence is not the main factor that determines patients’ lens selections, according to study investigators.
Ahmad Aref, MD, and Paul Singh, MD, offer a recap of their EyeCon 2021 presentation.
Ophthalmologists continue to rise to the challenge amid myriad hurdles.

Real-world evidence is growing in importance as a source of information that can help support clinical decision-making when evaluated properly.
Hear what Sumayya Ahmad, MD, and Jai G. Parekh, MD, MBA, have to say about current concepts for the management and treatment of dry eye.

The company anticipates that the Sirius and Celeste studies will further validate QR-421a’s ability to stabilize vision loss in people with USH2A mediated retinitis pigmentosa and Usher syndrome.
The US Orphan Drug Designation would provide seven-year market exclusivity if Visomitin was approved for use in LHON.

Researchers at the University of Virginia School of Medicine have made a discovery linking lupus, a potentially debilitating autoimmune disorder, and macular degeneration, a leading cause of blindness.