
The setting for this year's Ophthalmology Times® EyeCon 2022 at the JW Marriott Marco Island Beach Resort in Florida will provide the opportunity for clinical interaction between faculty and attendees along with the practicality of information.

The setting for this year's Ophthalmology Times® EyeCon 2022 at the JW Marriott Marco Island Beach Resort in Florida will provide the opportunity for clinical interaction between faculty and attendees along with the practicality of information.

The FDA describes the test as a web-based, self-guided software application that consumers can access using a touchscreen mobile device and internet-connected computer.

A team of researchers reported that oral doxycycline was effective, at least in the short term, for treating patients diagnosed with mild thyroid-associated ophthalmopathy.
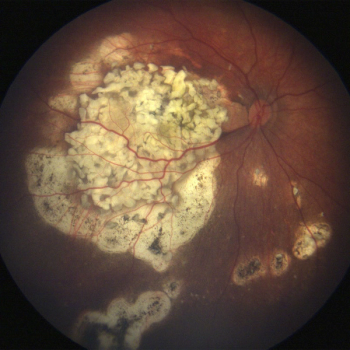
Children who develop the pediatric eye cancer retinoblastoma often lose their vision or an eye due to a lack of specific, targeted therapies and a poor molecular understanding of the cancer. Researchers at UT Southwestern and the University of Miami have discovered that a molecule – estrogen-related receptor gamma, or ESRRG – becomes hyperactive and promotes tumor cell survival in retinoblastoma.

New research paves the way for accurate non-invasive screening test that doe not have to be done in the clinic.

Leading U.S. eye care experts advance eye health focused digital platform designed to make eye care part of daily self-care routine.

Those who used both traditional and electronic cigarettes reported severe to very severe ophthalmic symptoms.
DME showed small significant improvement in study cohort.
The AVONELLE-X long-term extension study will continue to evaluate the efficacy, durability, and safety of faricimab in patients with neovascular AMD.
The data analysis indicated that patients achieved robust visual gains and central subfield thickness reductions with faricimab dosed every 8 weeks and with PTI dosing up to every 16 weeks. The gains were sustained through year 2 of the trials.

According to a Vanderbilt University news release, the International Retinal Research Foundation has extended a $10 million gift to establish a center dedicated to retinal vision research at the Vanderbilt Eye Institute and a directorship to support a physician-scientist leader in the Department of Ophthalmology and Visual Sciences.

During the American Academy of Ophthalmology 2022 Annual Meeting in Chicago,Tarsus Pharmaceuticals Inc. announced the launch of the “Look at the Lids” disease education campaign for Demodex blepharitis.

Optimal performance has a limited window.

AB 2236 would have allowed optometrists to perform anterior segment laser and minor procedures which involve the use of a scalpel or injections.

Library of ViewPoint drug candidates will accelerate development of novel alpha-crystallin chaperone compounds as a non-surgical treatment for age-related cataracts.

The survey, from The Larry A. Greene Center and the Primary Care Collaborative, found that the COVID-19 pandemic has had an adverse impact on the well-being of physicians.

On this week's episode of the NeuroOp Guru, Andy Lee, MD, and Elizabeth Fortin, MD, discuss visual snow syndrome and whether it should be evaluated during standard in-clinic ophthalmologic testing.

The hour-long issue teaches the viewing ophthalmologist the technique and many modifications of intrascleral haptic fixation, an innovative approach when the capsule has been damage or is absent.
After a brief hiatus during the pandemic, the Flying Eye Hospital program returns.

The destination of Marco Island, Florida not only serves as an excellent backdrop for this year's Ophthalmology Times® EyeCon 2022 but also provides a unique opportunity for family members to see their ophthalmic loved ones in a professional light.

Let us know if you will plan to use biosimilars in your daily practice. The poll will be open until October 10, 2022.

The American Academy of Ophthalmology will hold its annual meeting beginning Friday at McCormick Place in Chicago.

The Florida Second District Court of Appeal has renewed a woman’s lawsuit claiming she suffered permanent eye damage when her ophthalmologist misdiagnosed her during nasal surgery. In its opinion, the appellate court ruled that the trial court erred when it dismissed the lawsuit.

Recent reports of retinal atrophy have raised concerns on potential long-term safety.
The advanced swept-source OCT technology allows visualization of the variations in the normal changes and those associated with aging in the posterior vitreous.

The treatment of vision problems can help improve the quality of life for many people in the city.
Jeremy Tan, MD, discusses the latest trends in eye trauma surgery at the Dean McGee Eye Institute.

NYU Langone Health has been recognized as No. 1 in quality and safety for inpatient and ambulatory care by Vizient Inc.
Co-chairs Peter J. McDonnell, MD, director of the Wilmer Eye Institute at Johns Hopkins University School of Medicine in Baltimore, Maryland, and Oluwatosin U. Smith, MD, of Glaucoma Associates of Texas in Dallas break down the top reasons to attend this year's Ophthalmology Times® EyeCon 2022 in a series of video segments.

Provectus will have an exclusive worldwide license of intellectual property developed by the Ophthalmic Biophysics Center of the Bascom Palmer Eye Institute.