
According to the company, the OK-101 phase 2 trial will incorporate primary and secondary efficacy endpoints characterizing signs and symptoms of dry eye disease.

According to the company, the OK-101 phase 2 trial will incorporate primary and secondary efficacy endpoints characterizing signs and symptoms of dry eye disease.
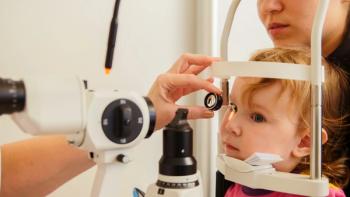
The case series included 12 eyes of children with refractory childhood glaucoma who needed trabeculectomy or implantation of a glaucoma drainage device.

According to the company, the updated PDUFA goal date is February 26, 2023. The FDA also restated that they do not plan to hold an advisory committee meeting to discuss the application.

LBS-008 is an orally administered tablet intended as an early intervention to slow disease progression in patients affected with Stargardt disease and dry age-related macular degeneration.

Diabetes can damage the eyes over time and cause vision loss, even blindness. Delaying the disease can also delay any onset of vision problems, including diabetic retinopathy and diabetic macular edema.

With a personal connection to glaucoma, Hanna and Mark Gleiberman establish a new center and three endowed chairs to build upon the university’s renowned vision institute

When it is not on a mission, the Orbis Flying Eye Hospital MD-10 aircraft often is situated at an airport where the fully accredited teaching facility is put to use as a mobile simulation center to train ophthalmologists from around the world in some of the latest sight-saving techniques.

As the Swiss company pivots to focus on core therapies, a report claims it is considering selling off its ophthalmology and respiratory units, with ophthalmology alone expected to bring $5 billion.
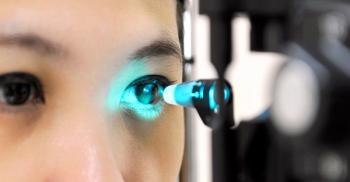
Company officials will drive global commercialization strategy for the company’s novel, non-invasive micron-accurate, OCT-guided femtosecond laser procedure for the treatment of glaucoma.

Prevent Blindness will provide TED educational materials, promote awareness, and offer unique art therapy program for patients and care partners.

By placing the chin on the chinrest, Nidek’s NT-1p automatically detects the position of the eyes and begins measurement without pressing any buttons.

While the advent of anti-VEGF therapies resulted in substantially better outcomes in this patient population, the results can vary substantially among patients.

According to UC Davis researchers, more needs to be done for the field to reflect ethnic and racial diversity across the country.
According to the university, the grant will support examining impact of pain and inflammation on eye’s surface and possible link to diseases.

According to the company, the multicenter US study enrolled more than 900 subjects with moderate-to-severe dry eye disease with topline data anticipated in the second quarter of 2023.
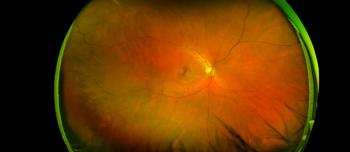
Mediwhale, an AI diagnostics company, aims for FDA approvals to increase non-invasive, early detection to save lives. Reti-Intelligence uses a simple fundus camera to capture images of the eye, and then within one minute the AI algorithm provides the disease risk assessment.

This December, the Ophthalmology Times® EyeCon 2022 at the JW Marriott Marco Island Beach Resort in Florida will provide attendees with an end-of-year opportunity to meet their continuing medical education requirements. This activity has been approved for 12.50 AMA PRA Category 1 Credits™.

According to the company, compounded formulations may be appropriate to prescribe for patients with clinical needs that are unmet by FDA-approved drug products. The prescriptions will be dispensed through Harrow’s wholly owned subsidiary, ImprimisRx.
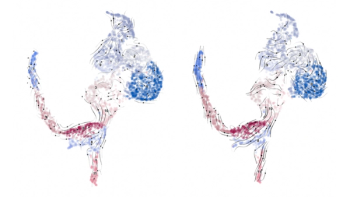
A new study of albino mice spotlights how the neural circuitry of vision is established, and how it can go wrong.

According to the company, the primary safety endpoint was achieved at all timepoints with all doses well-tolerated and no treatment-related our serious adverse events.
According to a study, use is linked to a lower prevalence of age-related macular degeneration in European populations.

The deal with FamyGem Life Sciences covers the development and commercialization of Nyxol eye drops for the reversal of mydriasis, presbyopia and night vision issues.

The company said it plans to generate clinical data aiming to demonstrate the potential retinal benefits of NCX 470 in order to add significant therapeutic value to the product profile in glaucoma.

Viatris intends to acquire Oyster Point Pharma as the foundation of its new ophthalmology franchise, recognizing its uniquely talented team, the strength of Tyrvaya nasal spray and its ophthalmology pipeline.

Every football fan knows the scrutiny that NFL game officials are put under every game day, and now a group of ophthalmologists is trying to ensure they can perform at their best each weekend.

According to investigators, findings may underscore the need for sleep therapy in patients at risk for glaucoma, and frequent eye checkups for patients who are poor sleepers.

According to Apellis Pharmaceuticals, the submission will be a Major Amendment to the NDA, extending the review period by three months with an expected PDUFA target action date in February.

Medicare’s 2023 fee schedule includes cuts in reimbursement, which some groups say could lead to reduced access to care to patients who need it most.

Jay S. Duker, MD, chief operating officer of EyePoint Pharmaceuticals, highlights the Phase 1 and Phase 2 DAVIO clinical trials for EYP-1901 in wet age-related macular degeneration. The company also announced the first patient has been dosed in the Phase 2 PAVIA trial of EYP-1901 for nonproliferative diabetic retinopathy.

OVDs that are currently used in cataract surgery fit into different classes including higher viscosity cohesives, lower viscosity dispersives, viscoadaptives and higher viscosity dispersives.