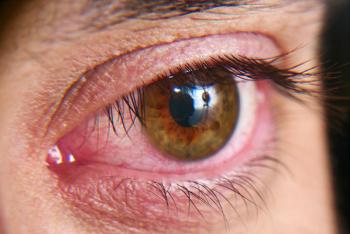
By creating customized packages that incorporate the range of diagnostic and treatment options available, practices can simplify dry eye management and improve patients' outcomes.

By creating customized packages that incorporate the range of diagnostic and treatment options available, practices can simplify dry eye management and improve patients' outcomes.

APOE4 gene associated with Alzheimer’s disease risk was found to protect mice from glaucoma. Research team also prevented retinal ganglion cell death by blocking the APOE signaling pathway, pointing to a potential treatment strategy for glaucoma.

Oyster Point Pharma Inc. today announced that the largest Medicare pharmacy benefit manager in the United States will add its varenicline solution nasal spray (Tyrvaya) for the treatment of dry eye on its Medicare Part D formularies, effective September 1.

A team of investigators has found that myopic refractive error is linked with an increased risk of primary open-angle glaucoma, and they indicate that the connection has a genetic foundation.
According to Johns Hopkins Medicine researchers, as little as $65 per year appears to influence practitioners, making them up to twice as likely to prescribe the companies’ brand name eyedrops for glaucoma instead of cheaper generic versions.

Researchers at Linköping University have used implants made from pig skin to restore sight to 20 people with diseased corneas.

Researchers at the Medical University of Vienna are focusing on how the retina can be used as a prognostic marker. Analyses revealed that retinal layer thinning as a result of an MS relapse predicts the severity of future relapses and the likelihood of disability.

Donald Salzberg, MD, conspired with an alleged accomplice at a medical diagnostic company in a scheme that ran for five years, from 2014 through 2019, according to U.S. attorney’s office in Boston.

The study provided evidence of safety, visual acuity improvement and structural stability in a difficult-to-treat patient population.
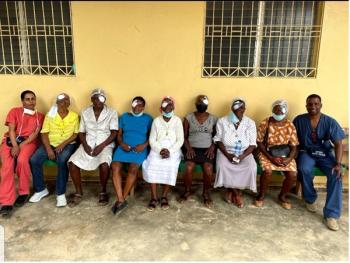
Anika Michael, MD, and Benjeil Edghill, MD, both ophthalmology specialists from AdvantageCare Physicians New York City, traveled to Haiti in July on a medical mission of mercy, where they evaluated and treated more than 400 patients and completed approximately 90 surgeries.

According to a news release, the U.S. Food and Drug Administration has approved Coherus’ ranibizumab-eqrn (Cimerli) as an interchangeable biosimilar for all five indications of Lucentis.

ThermaMEDx today announced a partnership with EyeCare Partners to expand EverTears distribution across the network of the national provider of clinically integrated eye care.

Combined, the companies say they will create the largest eye bank, tissue recovery and ocular research center in the world.

With the support of Harrington Discovery Institute at University Hospitals, an ophthalmic therapeutic dubbed KIO-301, initially developed by Richard Kramer, PhD, at the University of California, Berkeley (UCB), has successfully been granted approval to start a Phase 1b, first-in-human clinical trial.
Zachary Elkin, MD, MPH, of NYU Langone Eye Center, advises how ophthalmologists and parents should be aware of precautions that should be taken with sports involving small balls and urges the use of protective eyewear.

The gift will establish the Philip C. Hessburg, MD – Art Van Elslander Chair in Ophthalmic Research, which will constitute a permanent endowment fund to support the educational research initiatives at Henry Ford Health by the Detroit Institute of Ophthalmology.

Kodiak Sciences Inc. announced that its BEACON Phase 3 study of tarcocimab, its novel antibody biopolymer conjugate, met the primary endpoint of non-inferior change from baseline in visual acuity at week 24 compared to aflibercept in patients with macular edema due to retinal vein occlusion.

In a study, investigators found that no characteristics of inflammatory bowel disease are related to ocular manifestations of the disease.

According to the company, this Phase 1 dose escalations study will evaluate the safety and tolerability of intravitreal injection of IBI324 in subjects with DME.
At the recent ASRS meeting in New York, Aleksandra Rachitskaya, MD, of the Cleveland Clinic Cole Eye Institute, presented End-of-Study Retinal Fluid and Vision Outcomes in the Archway Phase 3 Trial of the Port Delivery System With Ranibizumab in Patients With nAMD.

The brain structures with most changes coincide with those which are altered most in Alzheimer’s, according to a study by researchers at the Universidad Complutense de Madrid in Spain.

Matthew Emanuel, MD, of Glaucoma Associates of Texas, discusses some of the current trends in cataract surgery. He notes it is something that everyone deals with as they get older. It also can be an issue for pediatric patients. Emanuel also notes that it is important for physicians to be able to educate their patients on the disease.

Tarsus Pharmaceuticals has enrolled the first patient in a Phase 2a clinical trial studying TP-03 for the treatment of meibomian gland disease in patients diagnosed with Demodex mites.

The project marks the Orbis Flying Eye Hospital's first return to in-person programming since the start of the pandemic.

The annual Mission Cataract program allows staff to extend its mission to people who need assistance due to unfortunate circumstances.
Retina indications for which ranibizumab-eqrn is interchangeable include neovascular (wet) age-related macular degeneration; macular edema following retinal vein occlusion; diabetic macular edema; diabetic retinopathy, and myopic choroidal neovascularization.

A novel regimen may represent an improvement over current methods of handling postsurgical pain in procedures that require corneal epithelial debridement, according to investigators.
Symptoms in the eye may not be substantial in patients with COVID-19.
Flavoprotein fluorescence could serve as a new biomarker, according to a Mount Sinai study.

Two studies conducted in collaboration with Orbis International explore how providing free glasses to children improves their ability to learn in school and that addressing visual impairment in children can alleviate depression and anxiety.