Several strategies exist to improve safety of injections for patients.

Several strategies exist to improve safety of injections for patients.

According to the company, it is planning to hold an end-of-phase 2 meeting with the FDA to discuss the results.

Viridian Therapeutics unveils positive data from its ongoing Phase 1/2 trial evaluating low dose VRDN-001 in patients diagnosed with TED.
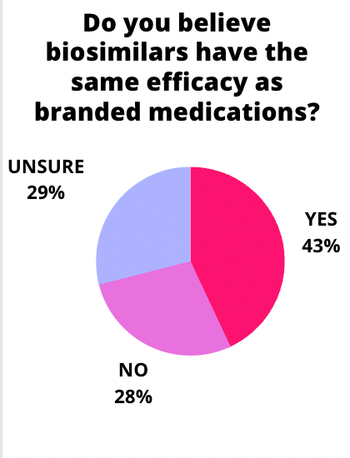
Results from our recent poll indicate a mixed bag of responses from ophthalmologists.

Rishi Singh, MD, of Cleveland Clinic Florida, touches upon the various advancements that are making a difference for clinicians and patients and reducing their progression in this disease state.

The deal adds pharmaceutical research and development capabilities and further expertise for future product pipeline. It also expands Alcon’s presence in the $20 billion global ophthalmic pharmaceutical category.

A recent study showed that only 6% of practicing ophthalmologists in the US identify as an underrepresented minority.

Ophthalmologists have learned to optimize their use of traditional anti-VEGF therapies and are now incorporating newer therapies like brolucizumab for managing retinal diseases, and in the not-so-distant future they can look forward to gaining familiarity with faricimab and using a new delivery system for ranibizumab.

Viatris intends to acquire Oyster Point Pharma as the foundation of its new ophthalmology franchise, recognizing its uniquely talented team, the strength of Tyrvaya nasal spray and its ophthalmology pipeline.

Jay S. Duker, MD, chief operating officer of EyePoint Pharmaceuticals, highlights the Phase 1 and Phase 2 DAVIO clinical trials for EYP-1901 in wet age-related macular degeneration. The company also announced the first patient has been dosed in the Phase 2 PAVIA trial of EYP-1901 for nonproliferative diabetic retinopathy.
Nicox reports that daily dosing of NCX 470 0.1% met the primary efficacy objective of demonstrating non-inferiority to latanoprost 0.005%.
This post-hoc analysis was performed to evaluate immunogenicity over the long term.

First-in-human biologic stent in ophthalmology is a conforming implant material, i.e., a soft, scleral wall compliant bio-tissue to structurally reinforce the cyclodialysis cleft opening.

The authors noted that the proportion of patients losing fewer than 15 letters from the baseline BCVA score in the study eye was comparable between the two groups.

According to Rishi P. Singh, MD, family history and lifestyle are key in the development of geographic atrophy.

Regeneron announced that the primary endpoints were met in two pivotal trials investigating novel aflibercept 8 mg with 12- and 16-week dosing regimens in patients with diabetic macular edema and wet age-related macular degeneration.
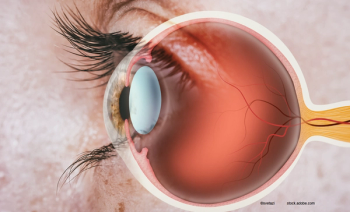
Retina indications for which ranibizumab-eqrn is interchangeable include neovascular (wet) age-related macular degeneration; macular edema following retinal vein occlusion; diabetic macular edema; diabetic retinopathy, and myopic choroidal neovascularization.

A novel eye drop under development may provide neuroprotection to the retinal ganglion cells.

According to the company, pegcetacoplan is an investigational, targeted C3 therapy for the treatment of geographic atrophy secondary to age-related macular degeneration.

Cynthia Matossian, MD, FACS, ABES, explains why eye care professionals should oversee the selection and use of artificial tears for patients with dry eye.

In the TENAYA and LUCERNE studies, more than 60% of faricimab patients could be treated every 4 months at 2 years, an increase from 45% at year 1. Study results are being presented at the American Society of Retina Specialists 2022 annual meeting in New York.
Kala Pharmaceuticals Inc. announced that it has completed the sale of its commercial portfolio and related intellectual property assets to Alcon Inc., a transaction that was first announced in May.
Lloyd Clark, MD, highlights his ASRS presentation focusing on the PANORAMA study detailing the use of aflibercept in the management of diabetic retinopathy.
David Brown, MD, highlights his ASRS poster that includes 44-week data for a four-times-higher dose of aflibercept in a real-world setting.
Rahul Khurana, MD, shares a preview of his ASRS poster based on an analysis of the second year of the VIEW 1 and VIEW 2 studies comparing aflibercept and ranibizumab treatment arms.