
The FDA has granted temporary discretion to import erythromycin ophthalmic ointment for the treatment of newborns.

The FDA has granted temporary discretion to import erythromycin ophthalmic ointment for the treatment of newborns.

Orasis Pharmaceuticals announces approval of Qlosi, a preservative-free, low-dose eye drop for presbyopia, which consistently demonstrated efficacy, safety, and tolerability in two pivotal Phase 3 trials.

Orasis Pharmaceuticals CEO Elad Kedar and COO Paul Smith sat down with David Hutton to discuss the FDA approval of Qlosi, a preservative-free low-dose eyedrops for presbyopia.

The data was presented in a poster titled at the American Thyroid Association (ATA).

Ocuphire Pharma and Viatris developed the drug together for the reversal of pharmacologically-induced mydriasis (RM) produced by adrenergic agonist or parasympatholytic agents.
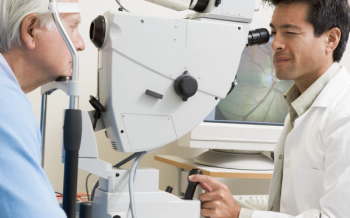
Preservative-free Iyuzeh, launched more than 10 years ago, currently is available in 46 countries, mostly under the brand name Monoprost, with about 1.5 million patients treated monthly, according to the company.
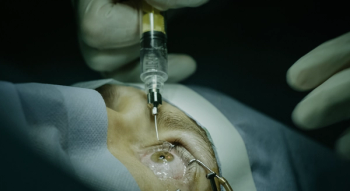
According to a news release, the J-code for SYFOVRE will become effective on October 1, 2023.
Sustained delivery strategy is offering hope for patients diagnosed with the disease.

Targeting the interleukin-11 receptor, LASN01 is a potential treatment for fibro-inflammatory diseases including thyroid eye disease (TED) and idiopathic pulmonary fibrosis (IPF).

In this podcast episode, Ophthalmology Times' co-chief medical editors Peter J. McDonnell, MD, and Neda Shamie, MD, delve into the clinical nuances of Demodex blepharitis. Through personal anecdotes, they stress its underrecognized gravity and discuss diagnostic cues like collarettes. The experts share their enthusiasm for improved treatments, echoing the transformative impact seen in dry eye care.

TOUR006 is an anti-IL-6 antibody with a differentiated profile for the treatment of thyroid eye disease (TED).
More than 135 patients have been recruited in 4 months for Phase 2 study of a single intravitreal (IVT) injection of ONL1204 ophthalmic solution as an adjunct to standard-of-care surgery.

The investigators performed a retrospective cohort study of patients from 10 US ocular inflammatory disease (OID) subspecialty practices.

A team of researchers have successfully transplanted human microglia into a mouse retina to create a model for studying eye disease treatments, such as diabetic retinopathy, glaucoma and age-related macular degeneration.

According to Regeneron, the approval was based on data in the PULSAR and PHOTON trials, in which the drug demonstrated clinically equivalent vision gains to aflibercept Injection 2 mg that were maintained with fewer injections.
According to the company, the MAA submission is based on data from the GATHER1 and GATHER2 Phase III clinical trials.

APP13007, if approved, may have an advantageous profile in dosing frequency and side effects while reducing the inflammation and pain associated with ocular surgery.

According to Regeneron, visual gains and safety of aflibercept 8 mg remained consistent with the established profile of aflibercept 2 mg injection.

According to the company, the therapeutic is the only approved GA treatment with a statistically significant reduction in the rate of GA progression at the 12-month primary endpoint across two Phase 3 clinical trials.

In a presentation at the American Society of Retina Specialists 41st Annual Meeting in Seattle, Diana Do, MD, said the PHOTON study demonstrated the impact aflibercept 8 mg could have in reducing the treatment burden for patients diagnosed with DME.

According to Tarsus Pharmaceuticals, lotilaner ophthalmic solution 0.25% (XDEMVY) is the first approved therapeutic for Demodex blepharitis, and has demonstrated efficacy across multiple clinical measures of disease.

The company also provided an update on tarcocimab development program for the treatment of neovascular age-related macular degeneration and diabetic macular edema.

With lawsuits looming over the issue, the FDA has updated the label on Horizon Pharmaceutical’s drug for thyroid eye disease to include risks associated with hearing loss.

According to the company, the transaction includes U.S. and Canadian commercial rights to six therapeutics, including two non-prescription brands.

A trial funded by the National Institutes of Health underscores need for more research to head off high myopia.