Retina
Latest News
Latest Videos

CME Content
More News

The companies will work together by using the codon-optimized BBS1 AAV9 vector to minimize the vision loss caused by the genetic defects in the BBS1 gene.
The AI-READI project aims to establish fair, equitable, and ethical access to big data, enhancing artificial intelligence’s ability to diagnose systemic diseases and drive progress in ophthalmology research.

The company states LYNX is the world’s first and only pattern scanning laser indirect ophthalmoscope and is battery-powered to provide surgeons with an untethered laser solution.

The investigators found that the premature births to be associated with vascular changes on ocular coherence tomography-angiography.

The NeurEYE research team will use millions of eye scans from Scottish optometrists to create the data set.
The treatment showed stability or improvement in the Diabetic Retinopathy Severity Scale (DRSS) and generally the drug was well tolerated.
The NPI-001 (N-acetylcysteine amide) tablets are a proprietary investigational therapy for the treatment of patients with retinitis pigmentosa.

Changes can result in bacterial contamination, loss of drug activity, and inflammation

Previously, Teva was established as the European commercialisation partner of FYB201, Formycon's biosimilar to ranibizumab (Lucentis).


ViGeneron also received approval for dose escalation in the ongoing phase 1b clinical trial.

The multinational, multicenter, prospective RESTORE Study is the 5-year follow-up study of the phase 3 clinical RESCUE and REVERSE Studies of lenadogene nolparvovec to treat vision loss from LHON due to the MT-ND4 mutation.
Lana Rifkin, MD, a uveitis specialist at Ophthalmic Consultants of Boston, shared insights into her work and her role as committee chair of the uveitis section for the upcoming EnVision Summit, which will be held from February 14 to 17, 2025, at the Caribe Hilton San Juan in Puerto Rico.
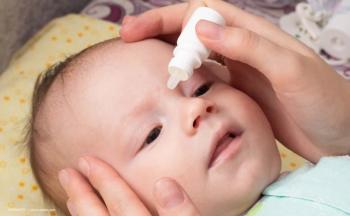
The rationale was that commercial mydriatics administered during retinopathy of prematurity (ROP) screening have been associated with cardiorespiratory and gastrointestinal adverse events.

Trending topics and milestone stories from our year in ophthalmology.

Look back on some of the top stories on Modern Retina from 2024 as we gear up for an incredible 2025.
A Helsinki University Hospital study found diabetes, glycemic control, or insulin therapy did not significantly affect anatomical or functional outcomes after epiretinal membrane surgery.

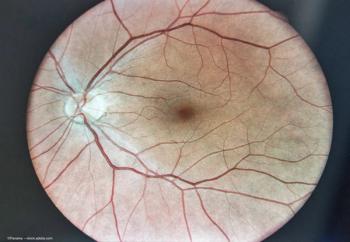
Enhancing durability and reducing treatment burden.

Investigators conducted a retrospective clinical audit to determine why patients do not adhere to the guidelines among about 9,000 patients in a practice.
The report details the results of preclinical discovery, engineering and characterization studies evaluating the safety, retinal cell transduction, transgene expression and clinical activity of proprietary evolved intravitreal vector R100 and 4D-150.

QUASAR is a global, double-masked, active-controlled phase 3 trial evaluating the efficacy and safety of EYLEA HD, compared to EYLEA, in patients with RVO, including those with central, branch and hemiretinal vein occlusions.

The company is advancing its Phase I/II trial and exploring accelerated approval pathways in the US and Europe.

A global survey explores the impact of geographic atrophy (GA) on quality of life, revealing similar challenges for individuals with unilateral and bilateral GA. Researchers also highlight the need for improved education, support, and treatment options.
In the LIGHTHOUSE study, Atsena Therapeutics is evaluating ATSN-201 gene therapy for X-linked retinoschisis, leveraging AAV.SPR capsid for central retina transduction without foveal detachment risks.