Retina
Latest News
Latest Videos

CME Content
More News

According to the company, the wearable smart glasses with both vision enhancement and assistance will be introduced at the American Retina Forum National Meeting

The service expands ophthalmological capabilities and enables providers to deliver the gift of sight to patients with complex retinal conditions in Harlem.
According to the researchers, the findings suggest drusen formation is a downstream effect of AKT2-related lysosome dysfunction and points to a new target for therapeutic intervention.
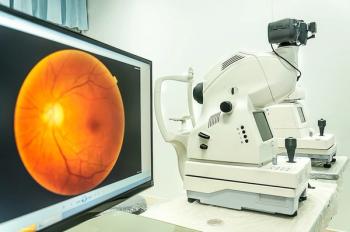
In a study, a team of researchers with the OhioHealth Grant Medical Center Family Medicine practice implemented diabetic retinopathy screening using in-clinic retinal photographs and automated software analysis to increase screening rates.

According to the company, VCN-01, its lead product candidate, is a systemic, selective, stroma-degrading oncolytic adenovirus. The FDA had previously granted orphan drug designation to VCN-01 for treatment of retinoblastoma.

KIO-30 is a small molecule photoswitch that selectively confers light-sensing capabilities to retinal ganglion cells following the degeneration of photoreceptors in inherited retinal diseases.

Nebraska Medicine taps technology for use in clinics.

The results from the trial in China met both the primary and secondary endpoints with no serious adverse events or new safety signals.

Lens feathering resulting from intraocular gas can interfere with the visualization needed to perform posterior segment surgery

Sruthi Arepalli, MD, spoke about her presentation, "Assessing retinal vascular changes in Alzheimer's disease with radiomics: A preliminary study of fundus photography" at the annual ASRS meeting in Stockholm, Sweden.

According to the company, OCU410 utilizes an adeno-associated virus platform for the retinal delivery of the RORA gene.

Therapeutics can prevent vision loss and neurodegeneration in patients.

The professor was recently given the award for his contributions to advancing vitreoretinal surgery, treatment, research, and patient care.
The purpose of the study was to determine if experience affects the surgeons’ dexterity response to pharmacologic and behavioral interventions.
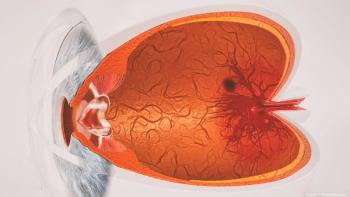
Scientists from the International Centre from Translational Eye Research used multiwavelength laser Doppler holography to assess blood flow in various layers of the human retina in vivo, which may impact the diagnosis of circulatory disorders.

Researchers, led by Shigemi Matsuyama, PhD, will conduct research seeking a potential breakthrough drug that can be taken orally—one that may address many RP disease manifestations, regardless of the underlying genetic mutation.

AAV204 is a novel adeno-associated virus (AAV) capsid from the AIM capsid library licensed by Abeona from the University of North Carolina at Chapel Hill.
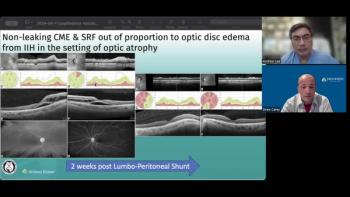
Andrew Lee, MD, and Andrew Carey, MD, sit down on another episode of the NeuroOp Guru to discuss nonleaking cystoid macular edema and subretinal fluid out of proportion to optic disc edema in idiopathic intracranial hypertension in the setting of optic atrophy.

The companies are teaming up to reduce care gaps and improve eye and vision health by bringing diabetic retinopathy and other eye disease screening services to patients in their own homes and workplaces.

The funding will go toward the continued clinical development of the company’s lead asset, AGTC-501 (laruparetigene zovaparvovec)
The multi-institutional found that a genetic variation common in people of African ancestry is linked with an increased risk of complications from diabetes, including diabetic retinopathy, which has previously been linked to genetic variations called single nucleotide polymorphisms, or SNPs, but these associations have been studied primarily in individuals of European and Asian ancestry.

The results were presented at a symposium during the American Diabetes Association's 84th Scientific Sessions in Orlando, Florida, and mark the first large-scale trial specifically designed to investigate the effect of fenofibrate on eye outcomes in people with early diabetic retinopathy.
Researchers at Washington University School of Medicine in St. Louis for years have worked to understand the rare condition known as retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations.

OCU410ST is a modifier gene therapy candidate being developed for Stargardt disease, which affects approximately 100,000 people in the United States and Europe combined.