Dry Eye
Latest News
Latest Videos

CME Content
More News

TFI employs spectral interference technology to image and map the corneal surface.

The primary endpoint will be pain improvement as measured by visual analogue scale (VAS) compared to placebo.

Most respondents were unsure of how to seek treatment and see dry eye as something that must be learned to live with.
Prevent Blindness is offering educational resources and will partner with OCuSOFT Inc. in support of Dry Eye Awareness Month in July.
Alice Epitropoulos, MD, and Laura M. Periman, MD, share insights on the various diagnostic tests they employ for patients exhibiting dry eye symptoms.
Physicians wonder whether normal examination results are actually normal.

Researchers found that blocking the interaction between peptide and receptor using topical applications of naltrexone reverses dry eye symptoms in 5 days and restores tear fluid volumes to normal baseline.

The trial is designed to enable a resubmission of a New Drug Application to the US FDA for reproxalap after receiving a Complete Response Letter last year.
According to the company, it will finalize its Phase 3 development plans following an End-of-Phase 2 meeting with the FDA.

The trial aims to evaluate the efficacy, safety, and tolerability of sterile ophthalmic ointment (AZR-MD-001) 0.5% compared to vehicle in patients with abnormal meibomian gland function (MGD) and associated symptoms of dry eye disease (DED)

According to the company, the data demonstrates sustained safety and efficacy in the treatment of the signs and symptoms in patients diagnosed with dry eye disease.

The gel is a cross-linked hyaluronic acid derivative and has been cleared by the FDA to temporarily block tear drainage by the occlusion of the canalicular system.

The launch of Blink NutriTears follows a clinical trial that demonstrated improved ocular symptom severity and tear film homeostasis, and that it helped tears stay on the eyes for 33% longer
InflammaDry is a diagnostic test that detects elevated levels of MMP-9, an inflammatory marker consistently found in the tears of patients with dry eye disease.
The trial is a study of twice daily tanfanercept ophthalmic solution 0.25% and 1.0% and will leverage key insights from the concluded Phase III VELOS-3 study.

Richard Adler, MD, FACS, offers key takeaways from a Case-Based Roundtable discussion that focused on the diagnosis and management of dry eye disease.

An expert on dry eye disease showcases how appropriate diagnostic tests play a valuable role in the diagnosis and management of dry eye disease.

Dr Adler offers nuanced clinical insights on the role of diagnostics in the management of dry eye disease.

Pucker shares topline results from a study that investigated the difference in meibomobian gland morphology when measured with a Visante OCT versus an OCULUS Keratograph 5M

Richard Adler, MD, FACS, reviews a patient case and discusses the ongoing discussion on how to define and identify dry eye disease.

The company expects to launch the supplement in the US, which features a proprietary blend of ingredients including lutein, zeaxanthin isomers, curcumin and vitamin D3, under the brand name Blink NutriTears early in the third quarter of 2024.

Analysis showed that patients with dry eye had “worse sleep quality than the healthy population."
Look beyond isolated treatment of the eye to address systemic factors.
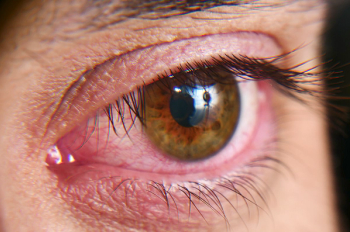
According to researchers, insights into the ocular microbiome could have implications beyond eye health.

The company said the module, which includes real-world data on more than 10 million patients, will help advance research and accelerate therapy development for dry eye disease and other ocular surface disorders.