A team of investigators has found that a comparison of ranibizumab and aflibercept showed both drugs safe and effective for treating diabetic macular edema.

A team of investigators has found that a comparison of ranibizumab and aflibercept showed both drugs safe and effective for treating diabetic macular edema.
According to the company, the trial did not demonstrate efficacy on the key clinical endpoints and will not advance THR-687 to Part B of the trial. As a result, Oxurion will now shift its focus to the Phase 2 development program for THR-149, which recently demonstrated a compelling safety and efficacy profile for the treatment of patients with DME.

Jennifer I. Lim, MD, FARVO, FASRS, reviews the 2-year results of the YOSEMITE and RHINE trials, outlining the efficacy, durability, and safety of faricimab in diabetic macular edema.

THR-687 is currently being evaluated in a phase 2 clinical trial in patients with DME. The first data from the dose-optimization study is expected to be released in the first half of 2022.

Riad Sherif, MD, CEO of Oculis, gives a company update on clinical trials for products in DME, ocular surgery, and more; plus, growing products in glaucoma treatments, team and financing updates.

According to the company, the trial will study the effect of the drug in treatment-naïve patients with diabetic macular edema (DME) who are members of underrepresented patient populations.

Raj Kannan, the new CEO of Aerie Pharmaceuticals, talks with Ophthalmology Times' David Hutton about what's coming down the pipeline for Aerie.


During a presentation at the Bascom Palmer Eye Institute Angiogenesis, Exudation, and Degeneration 2022 conference, Robert Bhisitkul, MD, PhD, provided 24-week clinical data from the Phase 1 study, demonstrating that patients with DME and wAMD showed improved visual acuity through 24 weeks following a single dose of UBX1325.

Dr. Katherine Talcott discusses baseline factors—CST thickness, hemoglobin A1C, and baseline vision—and their effects on DME resolution.

Arshad M. Khanani, MD, MA, told attendees at the Bascom Palmer Eye Institute’s 19th annual Angiogenesis, Exudation, and Degeneration 2022 Virtual Edition that data from the part A of the KALAHARI trial is encouraging, showing that THR-149 has the potential to improve vision in patients with DME who have a sub-optimal response to anti-VEGF therapies.
After 2 years, the improvements in vision and anatomy were sustained with extended dosing out to every 16 weeks in a high percentage of patients.

Faricimab is the first and only FDA-approved medicine targeting two distinct pathways, Ang-2 and VEGF-A, that often cause retinal diseases that may cause vision loss.

Joshua Mali, MD, shares how the FDA-approved faricimab-svoa (Vabysmo, Genentech) will change the treatment landscape for wet AMD and DME.

Across four studies, about half of eligible faricimab patients were able to go 4 months between treatments, and approximately three-quarters could be treated every 3 months or longer. Two papers published in The Lancet highlight one-year results.
Consecutive intravitreal dexamethasone treatments may be beneficial for patients with DME patients who had undergone a previous vitrectomy.
The improvement became evident during an average time of 6 months.

Approach shows potential as a promising second-line screening tool for patients with diabetes.

Real-world evidence is growing in importance as a source of information that can help support clinical decision-making when evaluated properly.

The company anticipates that the Sirius and Celeste studies will further validate QR-421a’s ability to stabilize vision loss in people with USH2A mediated retinitis pigmentosa and Usher syndrome.
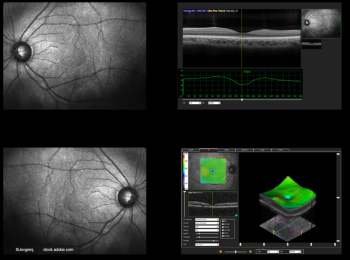
The difference in visual acuities based on CST fluctuations in these patients with diabetic macular edema remained significant.
SD-OCT is providing reproducible, high-quality, registered images to assess the treatment response in macular disease.
Investigators say renal function could be used as a possible predictor for poor treatment response in certain patients with diabetic macular edema.
Novartis has revealed the first interpretable results from year two (week 100) of the Phase III KESTREL study.
Patients’ reluctance to visit a clinic during the COVID-19 pandemic is pushing retina specialists to optimize therapy.