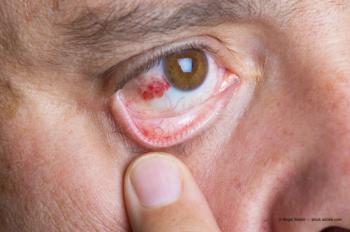
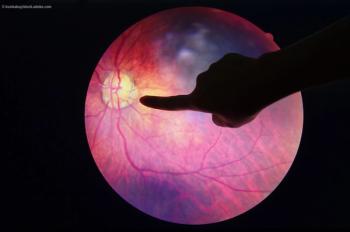
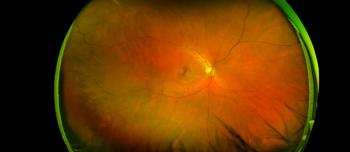
During the EnVision Summit Ophthalmology in Puerto Rico, Victor Villegas, MD, made a presentation titled "From a Retina Perspective: Comanagement of AMD, DME and CME in the Setting of Cataract Surgery," focusing on how to comanage patients that have macular degeneration, diabetic maculopathy and cystoid macular edema.