DME
Latest News

AiViva Biopharma completes enrollment in Phase 1 clinical trial of AIV007 for AMD, DME

Researchers uncover origin of phagocytes in the vitreous body of the eye
Latest Videos

CME Content
More News
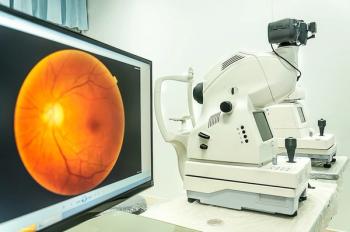
In a study, a team of researchers with the OhioHealth Grant Medical Center Family Medicine practice implemented diabetic retinopathy screening using in-clinic retinal photographs and automated software analysis to increase screening rates.

The results from the trial in China met both the primary and secondary endpoints with no serious adverse events or new safety signals.

AG-73305 is bi-specific, with anti-VEGF and anti-integrin. The dual mechanism of action has provided some interesting results.

EyeBio is developing a pipeline of clinical and preclinical candidates for the prevention and treatment of vision loss associated with retinal vascular leakage.

According to Genentech, faricimab-svoa is the first and only syringe prefilled with an FDA-approved bispecific antibody to treat retinal conditions that can cause blindness.

The biosimilar was approved for treating patients with age-related neovascular (wet) macular degeneration (nAMD) and other serious retinal diseases.
Durable treatments impact the quality of life of patients with retinal diseases.

According to the company, the acquisition includes 2 commercial assets Iluvien and Yutiq, expanding ANI’s foothold in ophthalmology.
The mapping method revealed prominent microscopic abnormalities consistent with diabetic retinopathy.

Low birth weight, blood transfusion, necrotising enterocolitis, bronchopulmonary dysplasia and antenatal steroid and surfactant therapies are among the factors that affect the development of ROP.

According to a study, the research reveals that boosting a specific protein, IRAK-M, in retinal cells could offer a new and highly effective therapy for AMD

Schocket has been serving as Interim chair since July, 2022, and during that time has worked to establish best practices for the department, strengthening its academic programs and improving its financial viability.

Mark Barakat, MD, sat down with Ophthalmology Times to discuss intraocular pressure outcomes with intravitreal injection of aflibercept, 8mg and 2mg in patients with diabetic macular edema through week 48 of the phase 2/3 PHOTON trial.
Understanding how the intricate networks of blood vessels in the eye and brain are formed ultimately could inspire new treatments for conditions like diabetic retinopathy and stroke.

Sean Adrean, MD, FAAO, sat down with Ophthalmology Times to discuss a post hoc analysis of the phase 2/3 PHOTON trial and the outcomes of patients with DME and baseline BCVA of 20/50 or worse or 20/40 or better who were treated with aflibercept 8 mg and 2 mg.

Deepak Sambhara, MD, sat down with Ophthalmology Times to discuss the impact of baseline central retinal thickness (CRT) on vision among patients with diabetic macular edema (DME), as a post hoc analysis of the phase 2/3 PHOTON trial.

The FDA recently approved Biologics' Yesafili as well as Samsung Bioepis and Biogen's Opuviz, both close copies of Regeneron Pharmaceutical’s Eylea. The approval offers ophthalmologists and patients additional therapeutic options.
The agency approved Yesafili (Biocon Biologics) and Opuviz (Samsung Bioepis, Biogen) as biosimilars to Eylea (Regeneron Pharmaceuticals).

The campaign will encourage, educate and empower the public to include vision and eye health as a part of their healthcare by asking them to share how eye care services and exams improved their vision and their daily lives.

While it fell short of the primary endpoint of improvement of non-proliferative diabetic retinopathy (NPDR) of at least 2 Diabetic Retinopathy Severity Scale (DRSS) levels as of week 36, DURAVYU did demonstrate stable or improved disease severity with reduced rates of NPDR in 9 months.
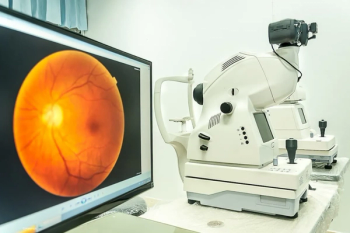
According to the company, 46.2% of patients demonstrated a 1- or 2-step improvement in the Diabetic Retinopathy Severity Scale (DRSS) at 40 weeks in the Axpaxli arm, compared to 0% in the control arm.

The molecule is being evaluated in 2 phase 3 clinical trials for wet AMD and is administered via intravitreal injection in combination with standard-of-care anti–VEGF-A therapy.

Oculogenex founder talks about collaboration with NASA and ISS on experiment in macular degeneration
Hema Ramkumar, an ophthalmologist and founder of Oculogenex, sat down with David Hutton of Ophthalmology Times to discuss their connection with NASA and their experiment on macular degeneration-treated mice in space.

Experts discuss the use of steroids, particularly in the context of diabetic macular edema (DME). The inflammatory nature of DME reveals the effectiveness of steroids, especially in cases where persistent fluid remains despite anti-VEGF treatment.



































