
The companies note that data from Study CPN-302 confirm the results in the first Phase 3 study, CPN-301, and they demonstrate the clear benefits of treating patients after cataract surgery with APP13007.

The companies note that data from Study CPN-302 confirm the results in the first Phase 3 study, CPN-301, and they demonstrate the clear benefits of treating patients after cataract surgery with APP13007.

Kodiak Sciences Inc. announced that its BEACON Phase 3 study of tarcocimab, its novel antibody biopolymer conjugate, met the primary endpoint of non-inferior change from baseline in visual acuity at week 24 compared to aflibercept in patients with macular edema due to retinal vein occlusion.

According to the company, this Phase 1 dose escalations study will evaluate the safety and tolerability of intravitreal injection of IBI324 in subjects with DME.

The brain structures with most changes coincide with those which are altered most in Alzheimer’s, according to a study by researchers at the Universidad Complutense de Madrid in Spain.

Tarsus Pharmaceuticals has enrolled the first patient in a Phase 2a clinical trial studying TP-03 for the treatment of meibomian gland disease in patients diagnosed with Demodex mites.

The project marks the Orbis Flying Eye Hospital's first return to in-person programming since the start of the pandemic.
According to the company, AR-15512 is a differentiated, novel, first-in-class product candidate for the treatment of the signs and symptoms of dry eye disease.

According to the company, the clinical trial is reviewing EYP-1901, an investigational sustained delivery anti-vascular endothelial growth factor (anti-VEGF) treatment for wet age-related macular degeneration (wet AMD).
Retina indications for which ranibizumab-eqrn is interchangeable include neovascular (wet) age-related macular degeneration; macular edema following retinal vein occlusion; diabetic macular edema; diabetic retinopathy, and myopic choroidal neovascularization.
Flavoprotein fluorescence could serve as a new biomarker, according to a Mount Sinai study.

Bausch + Lomb has made an equity investment in Sanoculis and has entered into an exclusive European distribution agreement for MIMS minimally invasive surgical procedure.

Positive results from recent pre-clinical studies support the potential of long-acting PASylated nomacopan to advance toward IND/IMPD for clinical trials in geographic atrophy (GA) in dry age-related macular degeneration (dAMD), a disease with no approved treatments.

Oyster Point Pharma Inc. this week announced the expansion of patient access to varenicline solution (Tyrvaya) nasal spray and provided an update on the commercial performance of the spray in the United States. According to the company, its expanded patient access programs include more eligible patients with dry eye disease.
Scientists at the Louisiana State University Health New Orleans Neuroscience Center of Excellence have developed a new, experimental human cell line from retinal pigment epithelial cells.

Treatment and reduces burden of care, providing a treatment option for patients diagnosed with diabetic macular edema.

MyMD Pharmaceuticals Inc. announced recently that it has entered into a material transfer agreement with Bascom Palmer Eye Institute of Miami, Florida to collaborate on a pre-clinical study using MYMD-1 as a potential treatment for traumatic optic neuropathy.

According to the company, the study assesses the safety and tolerability of a single dose of CLS-AX administered to patients diagnosed with wet AMD by suprachoroidal injection.

Trial is evaluating the use of the Technolas TENEO excimer laser for vision correction surgery for hyperopia with astigmatism.
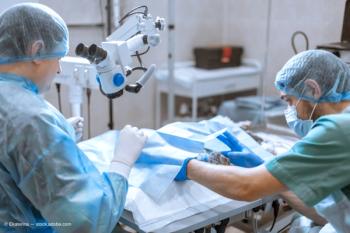
Results from the U.S. pivotal trial showed Apthera IOL subjects achieved statistically superior uncorrected intermediate and near vision, and equivalent distance vision and contrast sensitivity compared to control subjects.
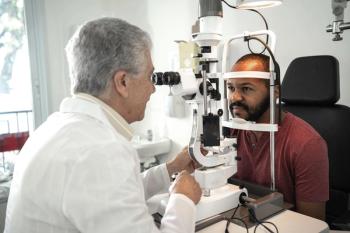
Mount Sinai study could lead to earlier and increased screening for this population to prevent blindness.

According to the company, 6-month safety and efficacy data are expected in Q1 2023. MCO-010 gene therapy reprograms healthy retinal cells to make them photosensitive.

LBS-008 is the company’s orally administered tablet for the treatment of Stargardt disease. There are currently no FDA approved treatments for Stargardt disease or dry AMD.
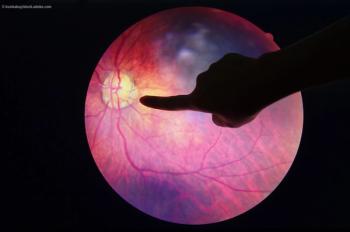
NIH-funded clinical trial finds that starting with a cheaper drug and switching to a more expensive drug as needed leads to good vision outcomes in diabetic macular edema.
According to a team of investigators from Yale, Stanford and Dartmouth, the variances in regional healthcare spending are not limited to either public or private payers.

The companies will jointly develop 4D bio-fabricated corneal transplants for diseases that require endothelial keratoplasty and natural lenticule transplants.

The ASRS See for a Lifetime See a Retina Specialist initiative will spotlight early detection and outline the importance of seeing a retina specialist.

According to the company, pegcetacoplan is an investigational, targeted C3 therapy for the treatment of geographic atrophy secondary to age-related macular degeneration.

A national poll suggests most parents overlook simple steps to protect children’s eyes; 1 in 7 parents say their child has not had a vision test in 2 years.

The company and its U.S. joint venture partner and licensee, HLB Therapeutics, signed a letter of intent this week with a global ophthalmology contract research organization to conduct a pair of phase 3 clinical trials simultaneously beginning in the fall in the U.S. and Europe for patients with neurotrophic keratitis.