
Nanoscope Therapeutics’ Phase 2 STARLIGHT open-label trial enrolled 6 subjects with advanced vision loss due to a clinical or genetic diagnosis of Stargardt disease.

Nanoscope Therapeutics’ Phase 2 STARLIGHT open-label trial enrolled 6 subjects with advanced vision loss due to a clinical or genetic diagnosis of Stargardt disease.

Haiyan Gong, MD, PhD, professor of ophthalmology and anatomy and neurobiology at Boston University School of Medicine, has received $200,000 through a Standard Award in National Glaucoma Research from the BrightFocus Foundation.

According to the company, the Phase 3 pivotal trials met pre-specified primary efficacy endpoints for both doses of iDose TR., supported an anticipated upcoming NDA submission.

Elisabeth J. Cohen, MD, has been appointed vice chair for academic affairs, enhancing the department’s commitment to research and advancing its reputation for excellence in studying and treating diseases of the eye.

If approved, TP-03 may offer treatment for millions of patients with Demodex blepharitis. TP-03 is now also being studied for the treatment of Meibomian Gland Disease in patients with Demodex mites.
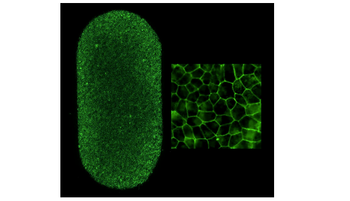
According to the National Institutes of Health, the therapy was derived from the patient’s blood by converting blood cells to iPS cells which were then programmed to become retinal pigment epithelial cells, which were surgically implanted as a patch of tissue.
GATHER2 met its prespecified primary endpoint of mean rate of growth (slope) in GA area at 12 months with statistical significance and a favorable safety profile. With this data in hand, Iveric Bio is working to submit a New Drug Application for Zimura to the FDA by the end of the first quarter of next year.
According to a presentation by the company at the 22nd EURETINA World Congress in Hamburg, Germany, the dataset shows that OCS-01 eye drops were more effective than vehicle in reducing central macular thickness and improving visual acuity in patients with DME as per the pre-defined criteria for statistical superiority in the study protocol.

A potential first-in-class eye drop with a novel mechanism of action, NOV03 is an investigational therapy to treat the signs and symptoms of dry eye disease associated with Meibomian gland dysfunction.
According to a university news release, its CellSight teams clinched top two of three awards in National Eye Institute competition.
According to the company, the fully connected environment, digital technologies and artificial intelligence tools enable optimization of patient care.

ONS-5010 is an investigational ophthalmic formulation of bevacizumab under development to be administered as an intravitreal injection for the treatment of wet AMD and other retinal diseases.

The company has filed an ophthalmic IND submission for the first treatment of ocular conditions associated with DNA damage.


This award totals $65,000 to support strategic planning implementation.

Gene therapy has partly restored the function of the retina’s cone receptors in two children who were born completely colorblind, reports a new study led by UCL researchers.

Stoke Therapeutics announced enrollment of the first patient in a prospective natural history study of people ages 8 to 60 who are living with autosomal dominant optic atrophy.

Sight Sciences Inc. is taking the wraps off Sion, a manually operated device used in ophthalmic surgical procedures to excise trabecular meshwork.

According to the company, results showed increased effects over time with pegcetacoplan, with treatment effect accelerated between months 18 and 24.

Alcon and Aerie Pharmaceuticals Inc. have announced the companies have entered into a definitive merger agreement through which Alcon will acquire Aerie.
According to the company, topical eye drops anti-TNFα agent licaminlimab (OCS-02) relieve persistent ocular discomfort in severe dry eye disease.

APOE4 gene associated with Alzheimer’s disease risk was found to protect mice from glaucoma. Research team also prevented retinal ganglion cell death by blocking the APOE signaling pathway, pointing to a potential treatment strategy for glaucoma.

Oyster Point Pharma Inc. today announced that the largest Medicare pharmacy benefit manager in the United States will add its varenicline solution nasal spray (Tyrvaya) for the treatment of dry eye on its Medicare Part D formularies, effective September 1.

A team of investigators has found that myopic refractive error is linked with an increased risk of primary open-angle glaucoma, and they indicate that the connection has a genetic foundation.

According to the company, a study shows the non-implantable minimally invasive glaucoma surgery effectively reduces IOP and reduces need for IOP-lowering medications in patients diagnosed with open-angle glaucoma.
An independent data monitoring committee recommends continuing study with a sample size target of up to 350 Patients. According to the company, no safety concerns have been identified and topline results are expected during the second quarter of 2023.
According to Johns Hopkins Medicine researchers, as little as $65 per year appears to influence practitioners, making them up to twice as likely to prescribe the companies’ brand name eyedrops for glaucoma instead of cheaper generic versions.

According to a news release, the U.S. Food and Drug Administration has approved Coherus’ ranibizumab-eqrn (Cimerli) as an interchangeable biosimilar for all five indications of Lucentis.

ThermaMEDx today announced a partnership with EyeCare Partners to expand EverTears distribution across the network of the national provider of clinically integrated eye care.

Combined, the companies say they will create the largest eye bank, tissue recovery and ocular research center in the world.