Therapeutics
Latest News
Latest Videos

CME Content
More News

At the CCOI-Stanford Summit, Goldberg calls for collaborative trial models that could redefine efficiency and standardization in ophthalmology research.
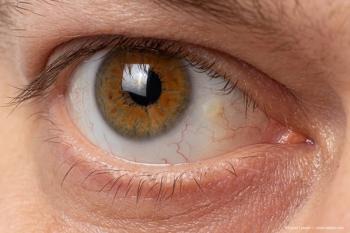
Pinguecula is a benign, common degeneration of the conjunctiva that appears as a grey-white-yellow mass on the bulbar conjunctiva.


In honor of Ophthalmology Times’ 50th anniversary, anterior segment surgeons attending ASCRS 2025 weigh in on the innovations that defined modern ophthalmology.
New technologies and a proactive mindset are transforming how glaucoma is diagnosed and treated—and redefining what “controlled” really means.
Intracameral tPA can treat fibrinous clots in severe anterior uveitis.
A look at current therapies, clinical trial results, patient education, and emerging options for managing this progressive retinal disease.
Timely diagnosis and personalized treatment are key to improving visual outcomes.
Amneal Pharmaceuticals received FDA approval for its prednisolone acetate ophthalmic suspension, a steroid eye inflammation treatment referencing Allergan’s Pred Forte, with a US launch planned for Q3 2025.

Harrow expects BYQLOVI to be commercially available in the fourth quarter of 2025.


On the heels of the drug's FDA approval, clinicians weigh in on acoltremon’s (Tryptyr) novel mechanism of action and its potential to address unmet needs in dry eye care

Weighing the benefits and trade-offs of preserved versus preservative-free glaucoma treatments is key to optimizing long-term patient care.

At the 2025 ASCRS meeting, Dr. Sarkisian presented 3-year data on the iDose TR from the FDA clinical trial.

Perfluorohexyloctane demonstrated rapid symptom relief in patients with dry eye, with effects reported as early as 5 minutes after dosing.

Discover how real-world evidence shapes cataract surgery practices, highlighting user satisfaction with enhanced monofocal IOLs at the 2025 ASCRS Annual Meeting.

Discover how dropless cataract surgery enhances patient comfort and outcomes, reducing the need for eye drops and improving recovery efficiency.

Glaucoma management is shifting toward earlier, drop-sparing interventions using sustained drug delivery and advanced laser technologies that improve outcomes and quality of life for both patients and physicians.

Chinese researchers reveal that low-level red light therapy may control myopia in children but raises concerns about retinal damage and cone density reduction.
ENTOD Pharmaceuticals launches Myatro XL, a 0.05% atropine eye drop, to effectively slow myopia progression in children, available by prescription in 2025.

Personalizing treatment options and setting realistic expectations are crucial.

The novel corrective eye drop can be prescribed through pharmacy partners BlinkRx or Medvantx

The recent agreement with Cipla adds to the list of partnerships Formosa has formed for APP13007.

Batoclimab is an anti-FcRn treatment being developed for a range of autoimmune diseases and active thyroid eye disease (TED).
The treatment was approved by China’s National Medical Products Administration (NMPA) and is China's first, and the world’s second, approved IGF-1R antibody drug.